Ligand binding
The ligand/receptor binding is the first key step in GPCR signaling. Revvity offers a range of ligand/receptor interaction solutions in both radioactive format and fluorescence assays with our proprietary HTRF-based Tag-lite technology.
The ligand/receptor binding is the first key step in GPCR signaling. Revvity offers a range of ligand/receptor interaction solutions in both radioactive format and fluorescence assays with our proprietary HTRF-based Tag-lite technology.
-
Tag-lite - Tag-lite is a non-radioactive TR-FRET-based solution which can be used to assess the pharmacology and pharmacodynamics of ligand/receptor interactions. The receptor of interest is labeled with cryptate in a way that does not alter receptor binding. The corresponding ligand is labeled with d2 acceptor. When the d2-labeled ligand binds to the cryptate-labeled receptor, it produces a HTRF signal.
In a competition assay, the introduction of a competing small molecule dislodges the d2-labeled ligand. Consequently, the ligand is pushed away and the signal stops.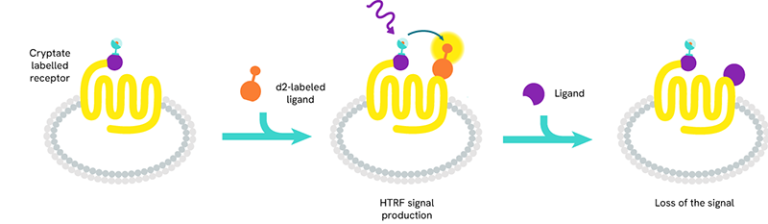
-
Receptor ligand binding with radiolabeled ligands and GPCR membranes - We also offer a range of radioactive ligands, which are used to study molecular interactions and QC our GPCR over-expressing cell lines and membrane preparations. Radiometric ligand binding assays are conducted on cells or cell membranes containing a GPCR receptor of interest. Radioligands can be used to perform saturation curves, competition, and kinetic experiments.
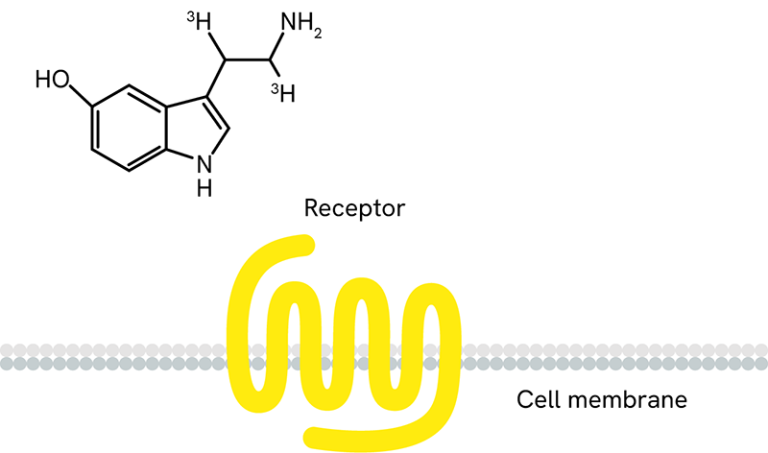
- Radioactive ligand binding assays can be performed in several different formats. Typically, we perform this assay in filtration format, where the unbound ligand is washed away using a vacuum manifold or cell harvester. The assay can also be conducted in a homogenous Scintillation Proximity Assay (SPA) format, where no wash steps are required.
Over 100 GPCR-expressing membrane preparations are validated for receptor ligand binding and over 50 are validated for GTPγS binding.
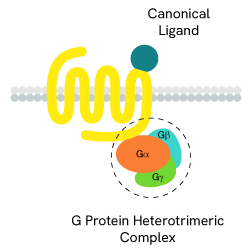
G-protein activation
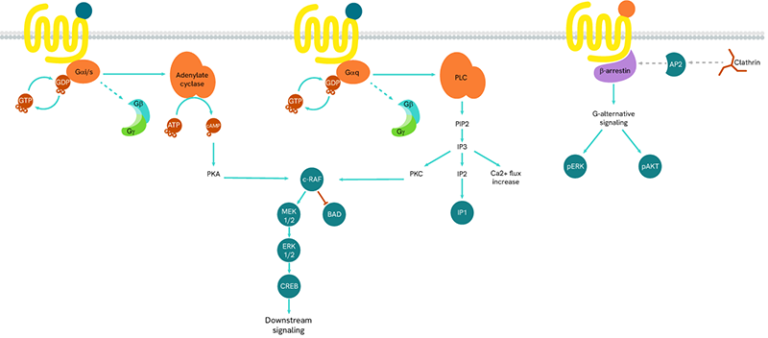
The main signal transduction of GPCRs is dependent on the receptor-mediated activation of heterotrimeric G-proteins.
The main signal transduction of GPCRs is dependent on the receptor-mediated activation of heterotrimeric G-proteins.
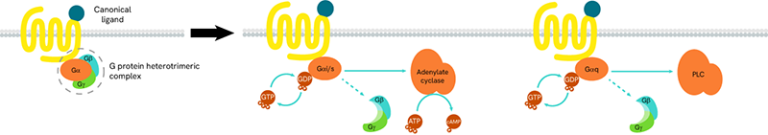
G-proteins are composed of three subunits (Gα, Gβ and Gγ) and are classified into four families (Gs, Gi/o, Gq/11, and G12/13):
- Gαs-coupled GPCRs positively stimulate the activity of adenylate cyclase, resulting in an increase in cellular cAMP
- Gαi-coupled GPCRs lead to a negative regulation of adenylate cyclase, and thus to a decrease in cAMP production
-
Gαq protein signaling is based on enzymes of the phospholipase C family (PLC).
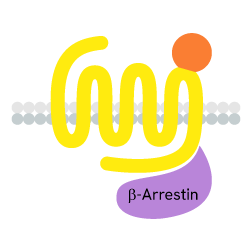
Arrestins recruitment
GPCRs also initiate G-protein-independent pathways that instead rely on arrestin coupling, which in turn suppresses G-protein activation.
GPCRs also initiate G-protein-independent pathways that instead rely on arrestin coupling, which in turn suppresses G-protein activation.
When a ligand binds to the extracellular part of a GPCR, the receptor undergoes conformational changes and opens its intracellular part to be phosphorylated by kinases. This phosphorylation of GPCRs by GRKs (GPCR kinases) is a prerequisite for high-affinity arrestin binding. The phosphorylated GPCR becomes a binding site for arrestins. Once linked, arrestins can interact with several partners such as AP2 or signaling proteins.

Intracellular signaling
GPCRs are at the beginning of many phosphorylation cascades involving phosphatases bound to second messengers.
GPCRs are at the beginning of many phosphorylation cascades involving phosphatases bound to second messengers.
Following the activation of a GPCR, signals are sent via G-proteins and arrestins to second messengers. Each GPCR has its own signaling pathway, but kinases and phosphatases are always involved and promote phosphorylation cascades to the nucleus. These molecular events are implicated in many physiological and cellular processes such as cell survival, proliferation, differentiation, and metabolism.
Featured resources