
Protein Clear HR Reagent Kit

Protein Clear HR Reagent Kit




The LabChip® GXII Touch Protein Clear™ HR assay is designed for the high-resolution protein purity assessment of monoclonal antibodies (mAb) within research and development, enabling the detection and visualization of impurities within a sample, down to 5 ng/μL.
| Feature | Specification |
|---|---|
| Instrument Compatibility | LabChip GXII Touch |
| Technology | Microfluidic Electrophoresis |
The LabChip® GXII Touch Protein Clear™ HR assay is designed for the high-resolution protein purity assessment of monoclonal antibodies (mAb) within research and development, enabling the detection and visualization of impurities within a sample, down to 5 ng/μL.


Protein Clear HR Reagent Kit


Protein Clear HR Reagent Kit


Product information
Overview
Protein Impurity Analysis in seconds
Achieve greater confidence in your quality control processes from development to commercialization with the LabChip® GXII Touch Protein Clear™ HR assay. The Protein Clear HR assay is designed for the high-resolution protein purity assessment of monoclonal antibodies (mAb) within research and development, enabling the detection and visualization of impurities within a sample, down to 5 ng/μL. With the analytical power and speed of the LabChip® GXII Touch system and IntelliChip™ assay optimization technology, you can accelerate your protein development workflows with unmatched throughput and reproducibility.
- Detect and visualize impurity peaks in 65 seconds
- Innovative IntelliChip technology provides intra- and inter-run reproducibility
- Baseline resolution of proteins as close as 0.2%, determined by mass spectrometry
- Limit of Detection (LOD) sensitivity of 5 ng/μL
- 21 CFR 11 compatible for manufacturing and QC workflows
Additional product information
The LabChip GXII Touch automated capillary electrophoresis platform delivers comparable results to traditional capillary electrophoresis with 46X faster run times without compromising sensitivity, resolution or reproducibility.
IntelliChip Assay Optimization Technology
The IntelliChip assay optimization technology automatically adjusts separation voltages and destain currents in real time to set optimal electrophoresis conditions for the sample plate, mitigating day-to-day variability, providing inter- and intra- run reproducibility in percent purity, and molecular weight sizing. A VeriMAb™ IgG standard is used during the calibration step as a reference standard to minimize the percent relative standard deviation (%RSD) for sizing and percent purity measurements.

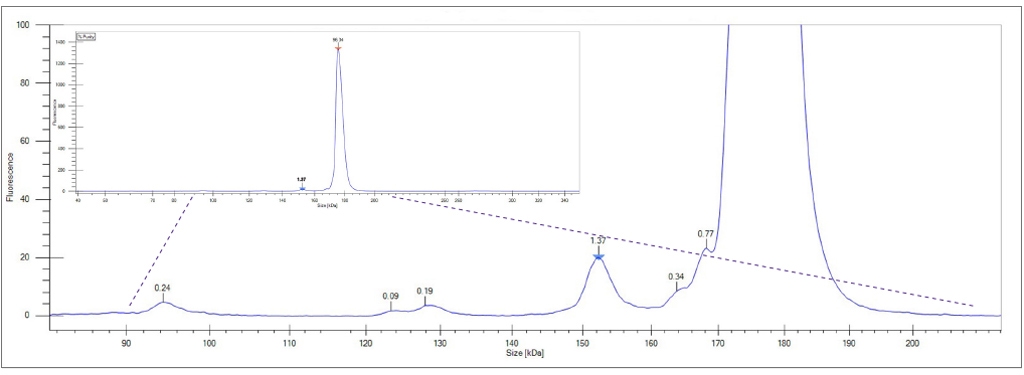
Figure 1. Protein Clear HR assay provides increased sensitivity and resolution to detect and visualize impurity peaks down to an LOD of 5 ng/μL. Inset electropherogram depicts complete run (65 seconds/ sample) of the Protein Clear HR assay for the non-reduced IgG mAb on the LabChip GXII Touch instrument.
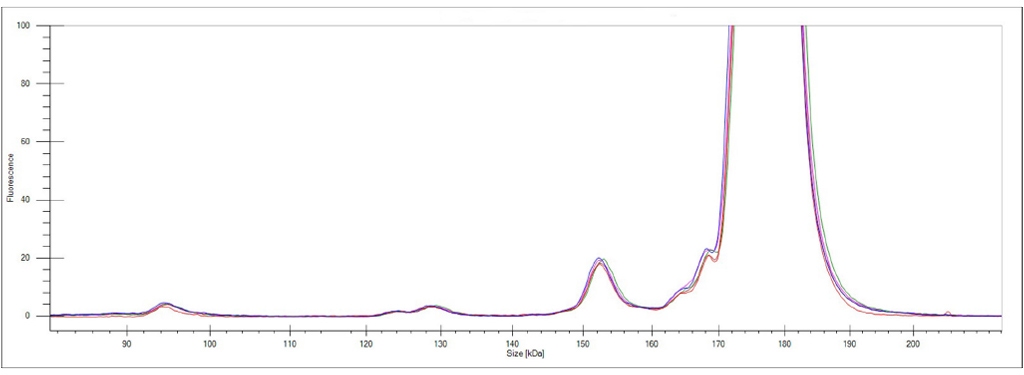
Figure 2. Overlay of five independent runs demonstrating the IntelliChip assay optimization technology inter-run result reproducibility.
21 CFR Part 11 Compatible
The LabChip GX Touch and Reviewer Software contains built-in technical controls and features specifically designed to be compatible with 21 CFR Part 11 requirements. These features include a shared user account database, access controls, device check, enforced sequencing of run steps, audit trails, record copying, record retention, system documentation, and electronic signature controls.
LabChip Touch instruments generate data records in electronic form which are archived in a Central Data Repository. The LabChip GX (GxP) Reviewer application can be run from any computer connected to the instrument network and allows modification and maintenance of the records with the ability to perform electronic signatures on the records generated from instruments in the lab system.
The robust and comprehensive LabChip GXP Software uses secured, computer-generated, time-stamped audit trails to independently record the date and time of operator entries and actions that create, modify, or delete electronic records (Figure 3). In addition, unique combinations of user ID and password for electronic signature are used. Once locked, a record cannot be further modified without a separate signed unlock action (Figure 4).
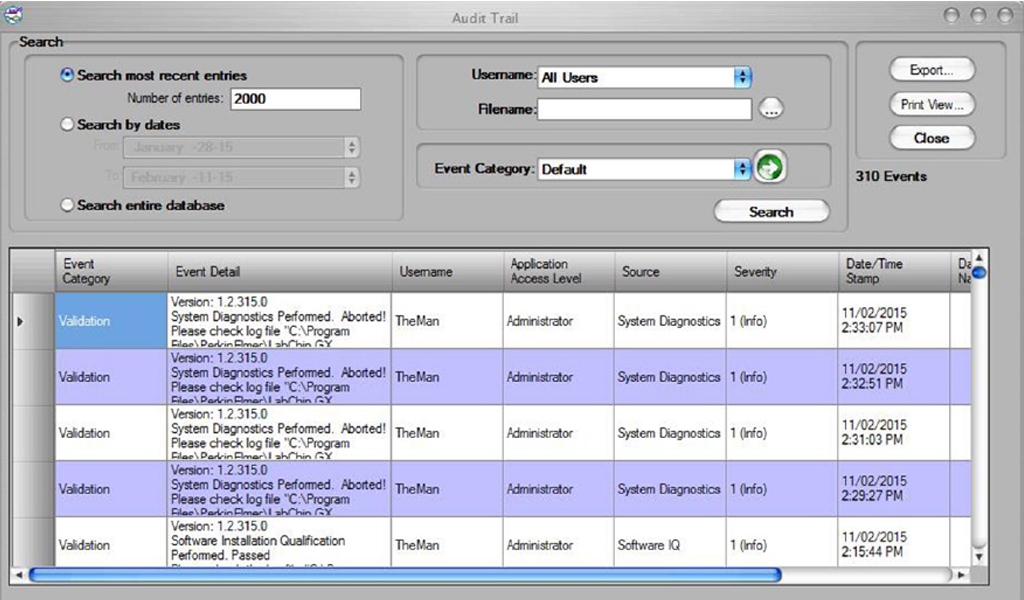
Figure 3. Audit Trails
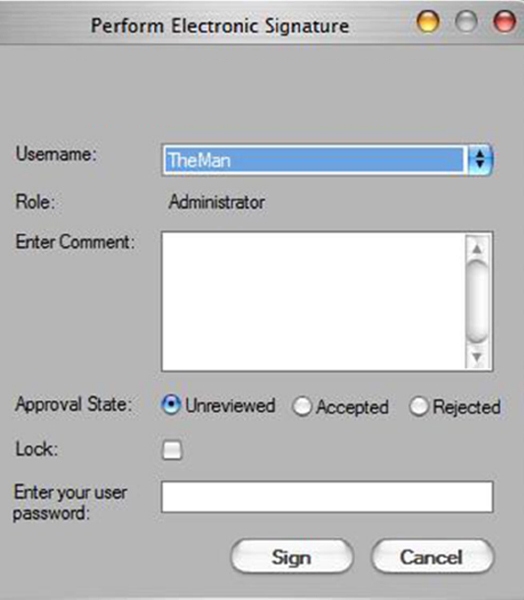
Figure 4. Perform Signature
System Requirements
Running of the Protein Clear assay requires a LabChip GXII Touch instrument with V1.6 software or newer and Data Reviewer V5.4 or newer.
Key Specifications
- Sizing Range: 14-250 kDa
- Linear Concentration Range: 10-1000 ng/μL (mAb, Non-reduced Main Peak)
- Linearity (R2): >0.995
- Sizing Resolution*: > 1.0 for VeriMAb Reference Standard
- Sizing Precision RSD (CV): <2% Maximum
- Relative Migration Time Precision RSD (CV): <2%
- Percent Purity Reproducibility: <0.5% Main Non-Reduced IgG; <5% All Other Peaks
- Sensitivity (LOD): 5 ng/μL (mAb, Non-reduced Main Peak)
- Separation Time per Sample: 65 Seconds
- Maximum Sample Concentration: 2000 ng/μL
- Number of Samples per Calibration: 96
- Chip Lifetime: 400 Samples
- Reagent Kit Primes: 10
- Minimum Sample Volume: 2 μL * Resolution is defined as the difference in migration times divided by the sum of the full width half max for two closely migrating peaks.
Specifications
| Application |
Protein Analysis
|
|---|---|
| Automation Compatible |
Yes
|
| Instrument Compatibility |
LabChip GXII Touch
|
| Sample Type |
Protein
|
| Shipping Conditions |
Dual Temperature
|
| Technology |
Microfluidic Electrophoresis
|
Configurations
Protein clear HR assay specifications
| Parameter | Specification |
|---|---|
| Sizing range | 14-250 kDa |
| Maximum sample concentration | 2000 ng/μL |
| Sizing resolution* | Resolution > 1.0 for VeriMAb reference standard |
| Sizing precision RSD (CV) | <2% |
| Relative migration time precision RSD (CV) | <2% |
| Percent purity reproducibility | <0.5% Main non-reduced IgG <5% all other peaks |
| Sensitivity (LOD) | 5 ng/μL (mAb, non-reduced main peak) |
| Linear concentration range | 10 – 1000 ng/ μL (mAb, non-reduced main peak) |
| Linearity (R^2) | >0.995 |
| Separation time per sample | 65 seconds |
| Maximum number of samples per calibration | 96 |
| Chip lifetime | 400 samples |
| Reagent kit primes | 10 |
| Minimum sample volume | 2 μL |
Resources
Are you looking for resources, click on the resource type to explore further.
In this application note mAbs were evaluated using both the Protein Clear™ HR assay and an orthogonal method, the SCIEX® PA800...
Read the LabChip® GXII Touch™ protein characterization system product note to learn about an automated alternative to traditional...
Capillary electrophoresis (CE), more specifically CE-SDS, is becoming an increasingly popular and routinely used method for...


How can we help you?
We are here to answer your questions.






























