
PG-Seq Rapid v2 kit

PG-Seq Rapid v2 kit




The PG-Seq™ Rapid kit v2 has been developed to analyze picogram quantities of DNA (single/multi-cells or low template DNA) from an embryo biopsy for preimplantation genetic testing. The PG-Seq™ Rapid kit v2 utilizes whole genome amplification (WGA) and next generation sequencing (NGS) technology to accurately screen all 24 chromosomes for whole chromosome aneuploidy and sub-chromosomal abnormalities. This PGT solution is compatible with Illumina® sequencing platforms, including the MiSeq® i100, as well as Element Biosciences® platforms.
| Feature | Specification |
|---|---|
| Product Group | PGTA Library Prep |
The PG-Seq™ Rapid kit v2 has been developed to analyze picogram quantities of DNA (single/multi-cells or low template DNA) from an embryo biopsy for preimplantation genetic testing. The PG-Seq™ Rapid kit v2 utilizes whole genome amplification (WGA) and next generation sequencing (NGS) technology to accurately screen all 24 chromosomes for whole chromosome aneuploidy and sub-chromosomal abnormalities. This PGT solution is compatible with Illumina® sequencing platforms, including the MiSeq® i100, as well as Element Biosciences® platforms.
Revvity is a trademark of Revvity, Inc. All other trademarks are the property of their respective owners.


PG-Seq Rapid v2 kit


PG-Seq Rapid v2 kit


Product information
Overview
- Fast, robust and easy library prep with PG-Find software included
- Up to 384 adapters for multiplexing on Illumina ® and Element ® sequencing platforms
- Detection of whole chromosome aneuploidies and segmental aberrations
- Possibility to detect sample swap and external contamination with mtDetect
Updated, streamlined, simple PGT protocol
From DNA to data, the PG-Seq™ Rapid kit v2 includes all reagents required for cell lysis, whole genome amplification, indexing along with the PG-Find™ analysis software for automatic calling of aneuploidy and copy number variants used in PGS (preimplantation genetic screening).
Now you can schedule a one-on-one 30-minute online demonstration with our experts to cover everything you need to know about the PG-Seq™ Rapid protocol and data analysis with PG-Find. Please choose a timeframe that suits you best and we will provide you a Microsoft Teams personal link to join when the time comes. We look forward to speaking with you.
Accurate Copy Number Detection
Extensively tested using over 100 cell lines and genomic DNA samples with known ploidy, including whole chromosome aneuploidies and segmental aberrations as small as 7 Mb. Demonstrated performance with as little as 30 pg of genomic DNA or 5-cell fibroblast samples, representative of a trophectoderm biopsy. See the PG-Seq™ Rapid Kit v2 application note for details.
Flexible Kit Format
The PG-Seq™ Rapid Kit v2 includes 48 reactions. Index sets are available for multiplexing from 48 to 384 samples, with all options available off the shelf. The larger 40 µL WGA PCR 1 reaction volume provides enough template for alternative downstream applications, such as PGT-M. It also delivers a high yield of 2–4 µg of DNA with just 23 PCR cycles.
Improved whole genome coverage and accuracy
Improved PG-Find quality scores compared to previous kit versions indicate reduced noise and bias, resulting in greater confidence. The PG-Seq™ Rapid v2 WGA PCR 1 product offers comprehensive whole genome coverage, making it compatible with hybridization capture panels.
Additional product information
mtDetect™ is a simple and intuitive tool that provides excellent internal control for sample traceability. It detects positional changes in embryos within the same run and compares the mitochondrial SNP profile in repeated biopsies with those of their siblings from previous runs, establishing the same origin of the samples. For us, this is a mandatory step before proceeding with sample analysis.
Dr. Marta Rodríguez de Alba
Jefe Asociado - Servicio de Genética
Hospital Universitario Fundación Jiménez Díaz (grupo QuirónSalud).
Sample Traceability in PGT-A
Sample traceability is crucial to ensure reliable embryo classification. This process involves tracking from the initial collection of the biopsy through to the final analysis.
For sample collection we recommend PCR tubes free of DNAse, Rnase, human genomic DNA and compatible with automation. Tubes with datamatrix code such as Cap2™ tubes, will facilitate sample tracking.
To track samples individually we take advantage of the mitochondrial genome.
The mitochondrial genome is maternally derived and contains single nucleotide variants that can be used to distinguish between individuals. Revvity has shown that correct grouping of sibling embryos can be achieved in >98% of cases using mitochondrial DNA SNV profiling. The new mtDetect™ web app extracts mitochondrial DNA information from data obtained during the standard PG-Seq™ Rapid v2 workflow enabling the monitoring of external DNA contamination and the ability to detect sample swapping and mislabeled samples.
Figure 1 shows 90% coverage of the mtDNA genome using the PG-Seq Rapid v2 kit without any protocol modification. Embryo biopsies processed with the PG-Seq™ Rapid v2 kit analyzed with mtDetect™ web app can successfully be identified and grouped based on their mitochondrial DNA SNV profiles. Identity determination allows sample tracking and can assist in the identification and monitoring of possible external sample contamination along with mislabeled samples.

Figure 1: IGV coverage track of the full mitochondrial genome (chrM: 1-16364bp) from a PG-Seq Rapid v2 amplified 5-cell sample with 1,000,000 sequencing reads shows 90% coverage of the mtDNA genome.
Easy-to-Use, Highly Customizable Analysis Software
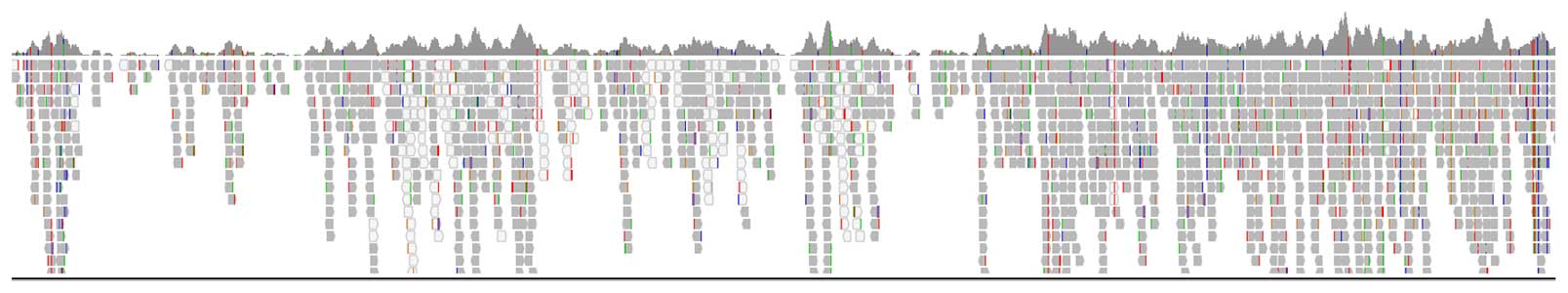
PG-Find supports self-reference and reference-based analysis options to suit different lab preferences. Includes flexible visualization tools—customizable chromosome display, raw vs. smoothed data toggle, multiple result image outputs, and unlimited color options. Users can adjust event calling thresholds to designate mosaicism as well as 1 or 2 copy gains and losses. A user-friendly overview screen enables quick interpretation of individual results and observation of broader data trends (Figure 2).
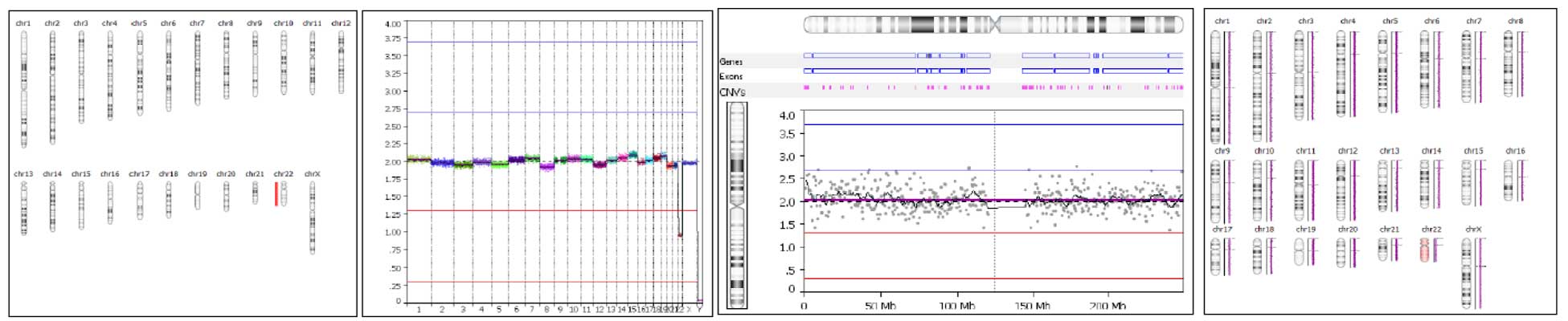
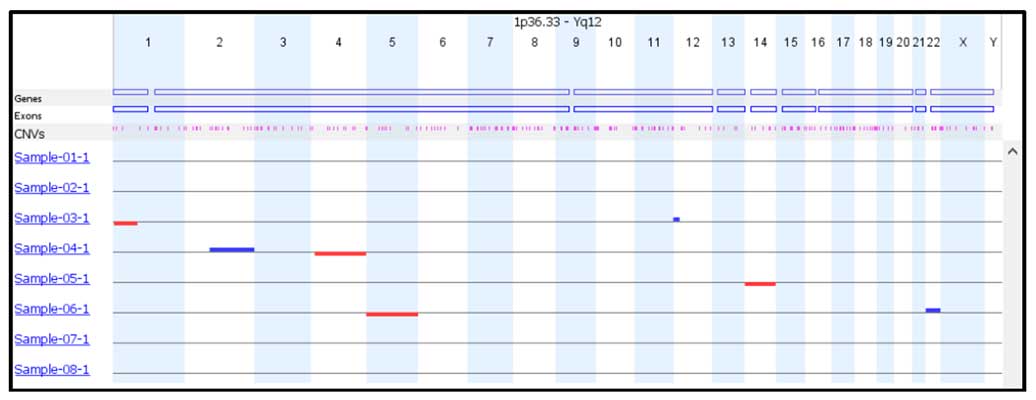
Figure 2. Examples of different visualizations that are used during routine analysis of data generated with PG-Seq™ Rapid v2
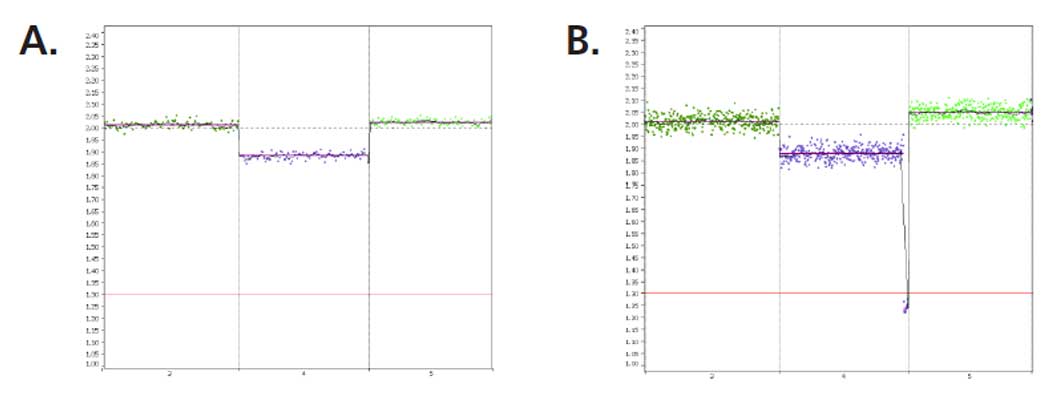
Figure 3. CNV resolution can be adjusted easily for each sample. Detailed information on the length and position of each even is provided.
Compatibility with Element® Biosciences sequencing platform
PG-Seq™ Rapid v2 libraries can be run native on the AVITI™ sequencer. Results obtained are concordant with those generated at the Miseq and no differences are observed in terms of quality and read per sample requirement at 10Mb resolution (Figure 4)
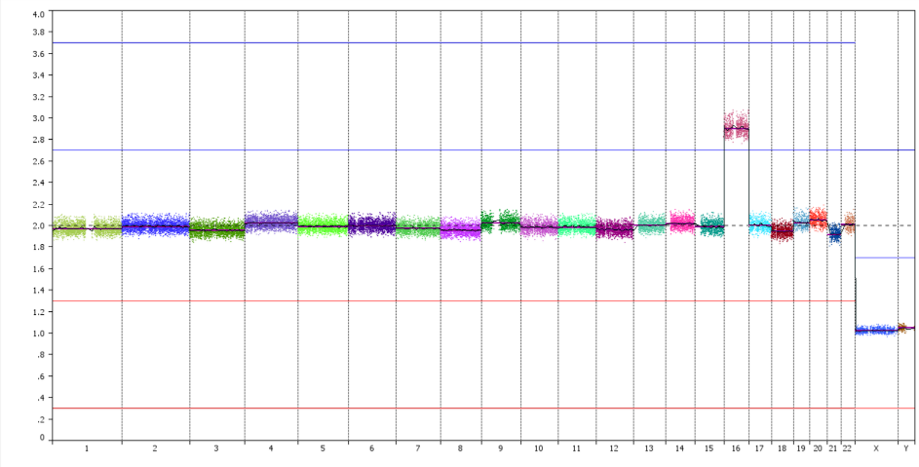
Figure 4: Example of data generated on AVITI from a GenQA sample (47XY, +chr16) and visualized at 10 Mb resolution.
Specifications
| Product Group |
PGTA Library Prep
|
|---|---|
| Shipping Conditions |
Dual Temperature
|
| Unit Size |
48 rxns
|
References
- Avila Perez, C., Parnell, L., Florensa Bargallo, M., Herreros Cuesta, J., Larreategui Laiseca, Z., Prados Dodd, N., Ruiz Perez, M., & Wells, D. (2023). P-731: The accuracy of truly non-invasive PGT using spent culture media is insufficient to justify routine clinical use. Human Reproduction, 38(Supplement_1), dead093. 1050.
- Banu, M., Pathan, A. A. K., & Chaitanya, K. V. (2023). Diagnostics for Genetically Inherited Disorders: From Cytogenetics to Genomics Technologies-A Review. Biomedical and Pharmacology Journal, 16(2).
- Cheng, H. Y. H., Chow, J. F. C., Lam, K. K. W., Lai, S. F., Yeung, W. S. B., & Ng, E. H. Y. (2023). Randomised double-blind controlled trial of non-invasive preimplantation genetic testing for aneuploidy in in vitro fertilisation: a protocol paper. BMJ Open, 13(7), e072557.
- Curnow, E., Ryan, G. L., & Yu, B. (2023). Discordant non-invasive PGT-A results and clinical outcome. Fertility and Sterility, 120(4), e276.
- Spath, K., Costa-Borges, N., Nikitos, E., Kostaras, K., Calderón, G. C., Psathas, P., & Wells, D. (2023). P-730: Detection of mitochondrial reversal following meiotic spindle transfer: a finding of importance for mitochondrial replacement therapies used for the purpose of avoiding…. Human Reproduction, 38 (Supplement_1), dead093. 1049.
- Sonehara, H., Matsumoto, R., Nakayama, N., Kobanawa, M., Numata, K., Kawasaki, A., & Shozu, M. (2022). Aneuploidy and sex concordance rate between cell-free DNA analysis from spent culture media of preimplantation embryo and DNA from whole embryo with respect to different…. Reproductive Medicine and Biology, 21(1), e12493.
Resources
Are you looking for resources, click on the resource type to explore further.
In this application note we detail the performance and accuracy of the analysis software on embryos analysed with the PG-Seq™...
This flyer showcases Revvity's end to end solution for PGTA.
When combined with the innovative Cap2™ tubes from Azenta® Life Sciences, the PG-Seq™ Rapid v2 kit provides anefficient and error...
The PG-Seq™ Rapid kit v2 offers analysis of whole chromosome and sub chromosomal copy number changes down to 7 Mb in size. The...
These instructions describe the process to prepare Illumina sample sheets for sequencing with the 8NT PG-seq primers.
These instructions describe the process to prepare Illumina sample sheets for sequencing with the 12NT PG-seq primers.


How can we help you?
We are here to answer your questions.






























