

HostDetect E.coli PCR DNA Quant Kit

HostDetect E.coli PCR DNA Quant Kit


| Feature | Specification |
|---|---|
| Analytical Sensitivity | > 0.03 pg/rxn |



Product information
Overview
The HostDetect™ E.coli PCR DNA Quant Kit is specific for DNA from E.coli genome. The reagents utilize sequence-specific primers and TaqMan® probe to amplify the 16S ribosomal RNA gene of E.coli genomic DNA for residual host genomic identification. A primer/probe set to detect Internal Control (IC), is also included for monitoring entire process from extraction to real-time PCR. The reagents also use a dUTP/UNG carryover prevention system to avoid contamination of PCR products. For more information, contact: BioprocessQC@revvity.com
Key Highlights:
- Quantify E.coli genomic DNA with as little as 0.03 pg per PCR reaction in < 2 hours of PCR
- Internal control detection for process monitoring from sample extraction to PCR
- High specificity: No cross-reactivity with unrelated DNA
- Flexible extracted DNA input volume up to 10 µL per well
- Scalable reaction numbers from 1 to up to 192 per kit
Additional product information
HostDetect™ E.coli PCR DNA Quant Kit Workflow

The probes for the E.coli genomic DNA and Internal Control detection are labeled with FAM and HEX/VIC fluorescent dyes, respectively, to generate target-specific signal. ROX is used for Passive Reference. Any Real-time qPCR Instruments with FAM™ and VIC®/HEX™ channels can be used with this assay.
HostDetect™ E.coli PCR DNA Quant Kit Quantification Range**
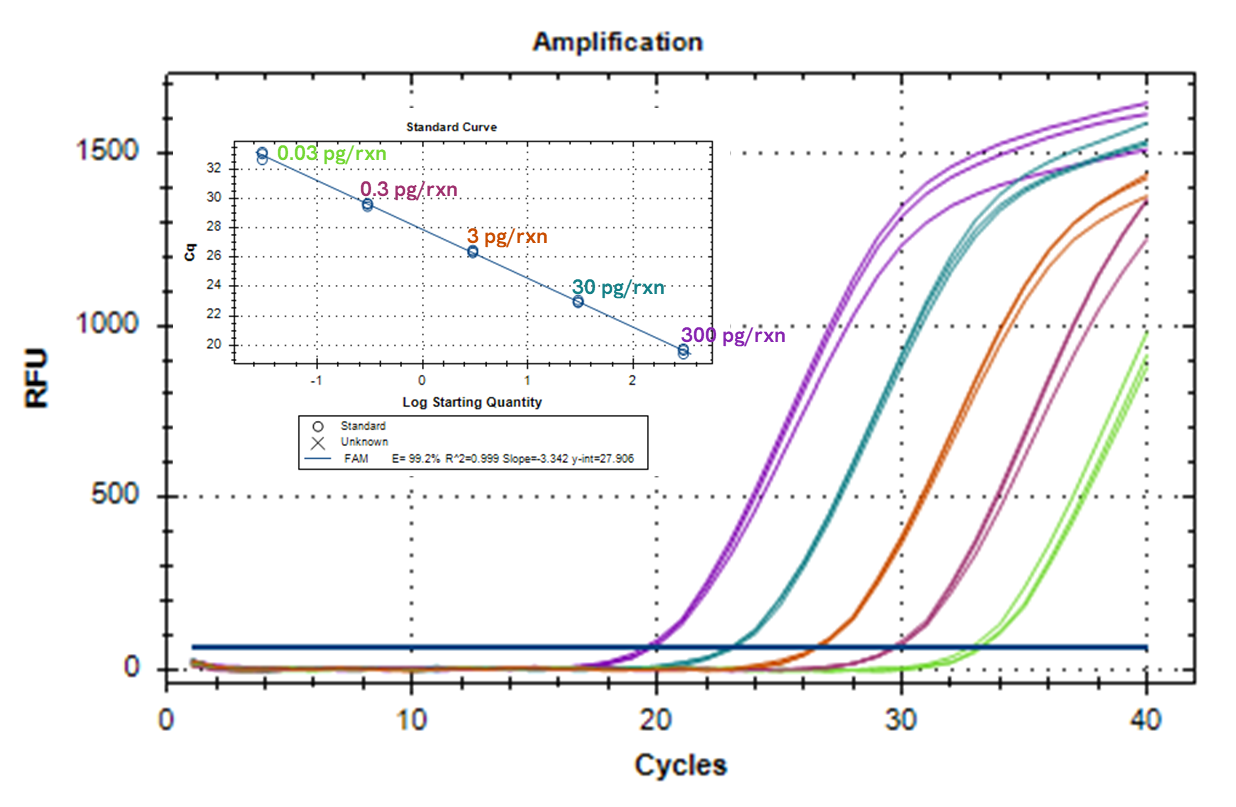
**: ATCC or USP reference standards for residual DNAs are not included in the kit. Customer must order these separately from ATCC or USP web sites.
Features:
| Quantification Range | 0.3 ng to 0.03 pg/rxn |
|---|---|
| Linearity | R2 ≥ 0.99, Eff%= 100±20% |
| Sensitivity | ≥ 0.03 pg/rxn |
| Precision | CV < 5% at 0.03 pg/rxn |
| Shipping Conditions | Shipped on Dry ice |
| Storage Conditions | -25 to -15 °C |
Specifications
| Analytical Sensitivity |
> 0.03 pg/rxn
|
|---|---|
| Shipping Conditions |
Shipped in Dry Ice
|
| Storage Conditions |
-25C to -15C
|
| Unit Size |
192 rxns
|
Resources
Are you looking for resources, click on the resource type to explore further.
"Residual HEK293 DNA is a critical impurity that must be minimized when generating recombinant AAV (rAAV) therapeutic products, as...


How can we help you?
We are here to answer your questions.






























