

Eonis Q96 (RUO)


Eonis Q96 (RUO)


Eonis Q96 (RUO)


The Eonis™ Q 96-well variant instrument has been designed to provide a simple and unique workflow, making training and implementation of qPCR easy to deliver. Due to the simplified workflow the Eonis Q boasts a less than 3-hour turnaround time, with no need for a clean room.
Product information
Overview
Eonis Q 96 is a real time PCR instrument with diverse possibilities to integrate into automation systems.
The Eonis Q instrument uses dried blood spot samples (DBS) samples punched from DBS cards. The DNA from the punches are extracted with a simple extraction protocol. No wash steps are required, only a 20 minute incubation in a simple incubator.
Eonis Q PCR cycler, that includes a dedicated LIMS compatible analysis software for the analysis and reporting of the results.
Additional product information
Eonis Q qPCR system and workflow
Screen for SCID and SMA
The adoption of newborn screening programs for SCID (severe combined immunodeficiency) and SMA (spinal muscular atrophy) are rapidly gaining momentum worldwide.
However, the integration and inclusion of new screening solutions into incredibly busy and crowded newborn screening labs often presents significant challenges.
This is why Revvity designed the Eonis Q workflow and system - to make SMA and SCID screening now a reality for labs of all sizes.

The Eonis Q system simplifies and streamlines molecular testing for SMA and SCID with an innovative dry chemistry workflow, comprising the Eonis Q96 instrument, the Eonis SCID-SMA kit and dedicated EASI software.

Punch samples
Single punch works for up to three analytes.

Elute
No wash steps required, saving your valuable time.

Analyze
Fast results of controls and samples.
With just three steps, workflow complexity is reduced, minimizing the need for specialist skills sets, while gaining fast and accurate results of controls and samples.

How we've simplified the Eonis Q dry chemistry workflow
- Reagents are dried on the plate: no need for a master mix preparation
- Multiplex from a single dried blood spot punch
- Quantification without calibrators
- Analyze over 500 samples per day
The Eonis Q is designed to be compact, offering a streamlined workflow with a swift 3-hour turnaround, eliminating wash steps and reducing the need for extensive pipetting and centrifuging processes.

EASI software
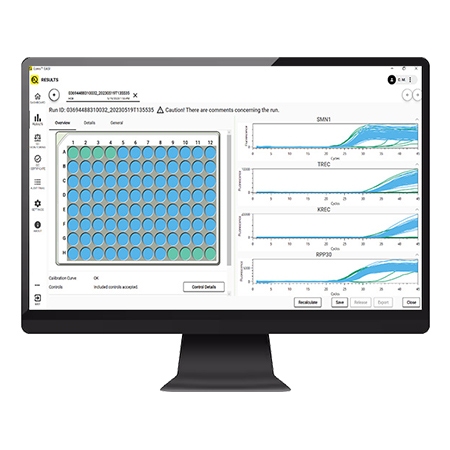
EASI Software a user-friendly dedicated newborn screening solution, streamlines processes with its easy-to-use interface, eliminating the need for prior qPCR software experience and additional tools.
EASI software seamlessly integrates with hospitals' LIMS, conducts QC monitoring for tracking over time, and offers trouble-shooting packages for effortless customer support collaboration.
Uniquely customized for newborn screening laboratories
- Easy to use without any previous experience of qPCR software
- The same software is used for managing the PCR run and analyzing the result
Seamless integration
- Connects to LIMS system easily with no extra tools needed to run the analysis
Time to report
- Fast reporting: no manual data handling, that requires extra time
Troubleshooting packages
- With just one click, you can easily share files with our customer support if you need assistance
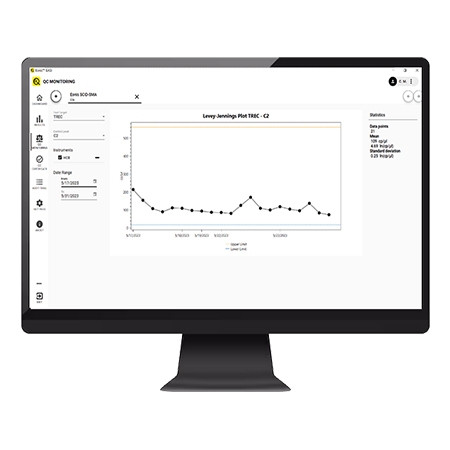
Quality control monitoring
- Built in QC monitoring ensures you can track your QC level over time: no need to keep any separate files for tracking
The Eonis Q system and workflow has been designed to make screening for SCID and SMA easier by optimizing time, space, cost and training.
Ordering information
Instruments
| Product name | Product number |
|---|---|
| Eonis Q96 instrument | 2044-0010 |
| Eonis EASI software | 2044-3010 |
| Eonis Q96 Dx EASI software licence | 2044-3020 |
| Eonis Q PC, monitor and barcode reader | 2044-8010 |
| 96-well optical test plate (for installation and PM only) |
X04-10-310P-105-14 |
| TriNEST microplate incubator shaker | 1296-0050 |
Reagents
| Product name | Description | Product number |
|---|---|---|
| Eonis SCID-SMA kit | 4x PCR plates; 96 reactions/plate, 88 samples/plate 1x bottle Elution Solution 2x Kit controls 1x Zero control |
3241-0020 |
Consumables
| Product name | Description | Product number |
|---|---|---|
| DNA elution plates | 20 plates | 4183-0010 |
| Adhesive foil seal | 100 plates | 4156-0010 |
| Optical PCR seal | 100 plates | 4197-0010 |
Specifications
| Dimensions | 310.0 mm (W) x 345.0 mm (D) x 613.0 mm (H) |
|---|---|
| Weight |
38.0 kg
|
| Accreditations |
RUO
|
|---|---|
| Application |
qPCR
|
| Brand |
Eonis™ Q
|
| Model |
Eonis™ Q96
|
| Unit Size |
1 each
|
Resources
Are you looking for resources, click on the resource type to explore further.
The EONIS Q is a real-time PCR instrument with possibility to integrate into automation systems.


How can we help you?
We are here to answer your questions.






























