
AlphaLISA CHO HCP Detection Kit, 5,000 Assay Points

 View All
View All
AlphaLISA CHO HCP Detection Kit, 5,000 Assay Points











The AlphaLISA™ immunoassay kit for CHO Host Cell Protein (HCP) Detection enables the quantitative determination of CHO HCP in buffer, cell culture media, and cell supernatants using a homogeneous AlphaLISA assay (no wash steps). The CHO HCP Detection Kit is also available in HTRF® format as an orthogonal technology.
| Feature | Specification |
|---|---|
| Application | Protein Quantification |
| Dynamic Range | 0.52 - 3,000 ng/mL |
| Limit of Detection | 0.52 ng/mL |
| Limit of Quantification | 1.80 ng/mL |
| Sample Volume | 5 µL |
The AlphaLISA™ immunoassay kit for CHO Host Cell Protein (HCP) Detection enables the quantitative determination of CHO HCP in buffer, cell culture media, and cell supernatants using a homogeneous AlphaLISA assay (no wash steps). The CHO HCP Detection Kit is also available in HTRF® format as an orthogonal technology.







AlphaLISA CHO HCP Detection Kit, 5,000 Assay Points







AlphaLISA CHO HCP Detection Kit, 5,000 Assay Points







Product information
Overview
CHO cells are widely used expression hosts for recombinant proteins and are utilized for the generation of monoclonal antibodies. The CHO Host Cell Proteins (HCP) kit is designed to measure contaminants originating from the CHO cells used to manufacture therapeutic antibodies. This kit can be utilized to quantify CHO HCP proteins at various stages, from routine bioprocess operations to crude harvest materials to final products. Our simple procedure and robust assay offer increased throughput compared to ELISA.
Formats:
- Our 100 assay point kit allows you to run 100 wells in 96-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 500 assay point kit allows you to run 500 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
- Our 5,000 assay point kit allows you to run 5,000 wells in 96-well or 384-well format, using a 50 µL reaction volume (5 µL of sample).
Features:
- No-wash steps, no separation steps
- ELISA alternative technology
- Sensitive detection
- Broad sample compatibility
- Small sample volume
- Results in less than 3 hours
- Half the time of an ELISA assay
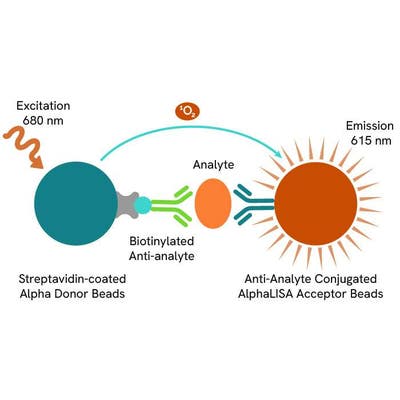
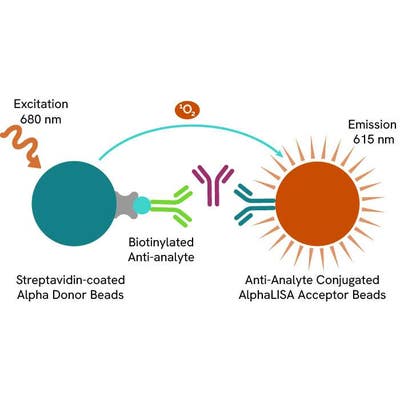
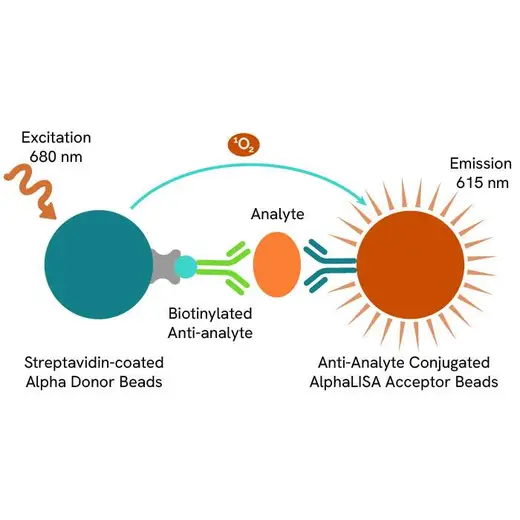
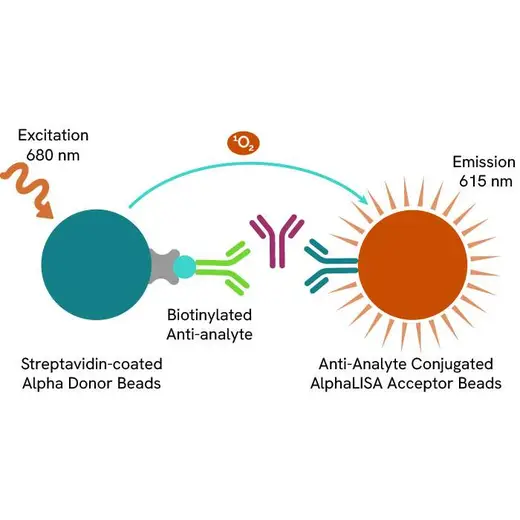
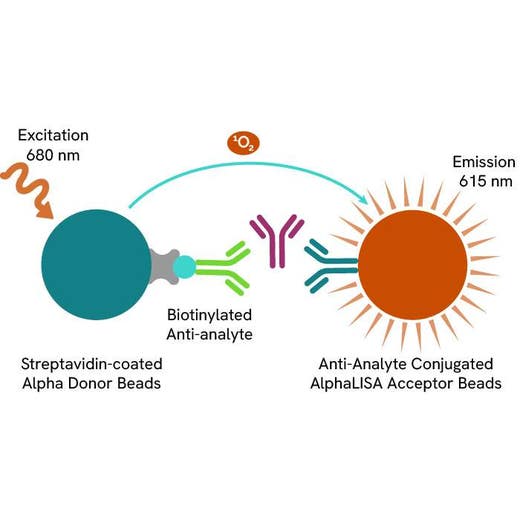
AlphaLISA technology allows the detection of molecules of interest in a no-wash, highly sensitive, quantitative assay. In an AlphaLISA assay, a biotinylated anti-analyte antibody binds to the Streptavidin-coated Donor beads while another anti-analyte antibody is conjugated to AlphaLISA Acceptor beads. In the presence of the analyte, the beads come into close proximity. The excitation of the Donor beads causes the release of singlet oxygen molecules that triggers a cascade of energy transfer in the Acceptor beads, resulting in a sharp peak of light emission at 615 nm.
Specifications
| Application |
Protein Quantification
|
|---|---|
| Automation Compatible |
Yes
|
| Brand |
AlphaLISA
|
| Detection Modality |
Alpha
|
| Dynamic Range |
0.52 - 3,000 ng/mL
|
| Limit of Detection |
0.52 ng/mL
|
| Limit of Quantification |
1.80 ng/mL
|
| Product Group |
Kit
|
| Sample Volume |
5 µL
|
| Shipping Conditions |
Shipped in Blue Ice
|
| Target |
HCP
|
| Target Class |
Biologics
|
| Technology |
Alpha
|
| Unit Size |
5,000 assay points
|
Image gallery






AlphaLISA CHO HCP Detection Kit, 5,000 Assay Points






AlphaLISA CHO HCP Detection Kit, 5,000 Assay Points






Video gallery

AlphaLISA CHO HCP Detection Kit, 5,000 Assay Points

AlphaLISA CHO HCP Detection Kit, 5,000 Assay Points

Resources
Are you looking for resources, click on the resource type to explore further.
Detecting and quantifying HCPs with automatable homogeneous immunoassays
During biotherapeutic manufacturing and production, the...
With over 200 different types of cancer, management relies on a variety of techniques such as chemotherapy, radiotherapy, and...
Unlock the future of therapeutics with multispecific antibodies
Discover the groundbreaking advancements in multispecific antibody...
Helping you put safety and product efficacy at the forefront of your GMP manufacturing
At Revvity we’re able to support your GMP...
Detecting and quantifying CHO HCPs with automatable homogeneous immunoassays
In recent years recombinant therapeutic proteins and...
Loading...


How can we help you?
We are here to answer your questions.






























