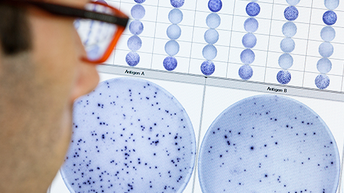
世界の結核の状況
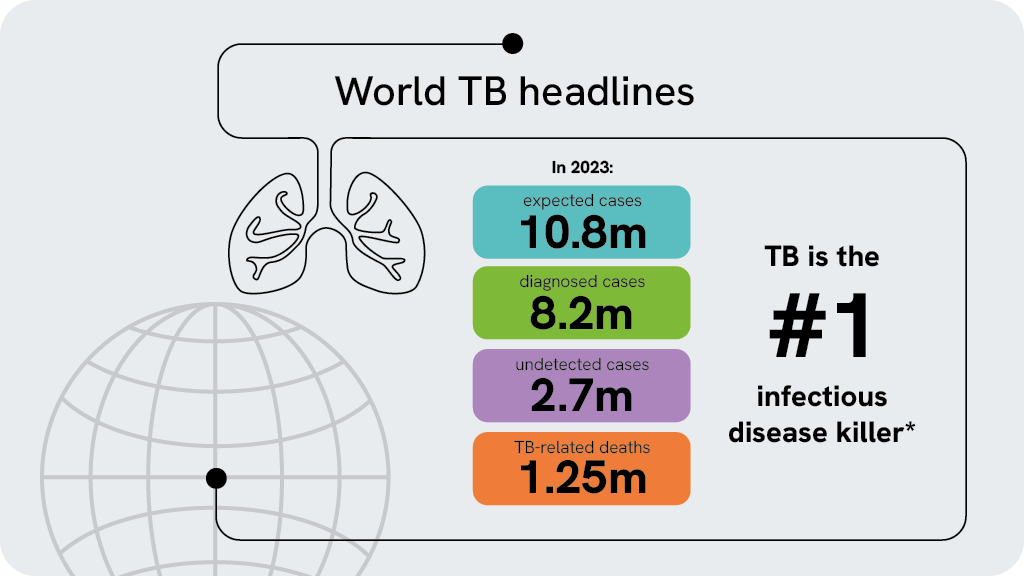
2023年だけでも1,000万人以上が結核にかかり、250万人がこの病気で亡くなりました。これは単一の感染源による死因として世界でも有数のものです1)。
活動性結核患者の背後には、はるかに多くの潜在性結核感染症(Latent tuberculosis infection: LTBI)患者がいます。LTBIは無症状であり、他人に感染させることはありませんが、感染者のうち5%から10%1)は、将来的に活動性結核を発病し、周りに感染を広げるリスクがあります3)。
このため、結核菌に感染しているLTBI患者を把握し、活動性結核を発病する前に治療を行うことは、世界的な結核対策において大きな意味を持ちます2),3)。
潜在性結核感染症(LTBI)の検査方法
潜在性結核感染症(LTBI)を見つける検査には、免疫反応を利用するツベルクリン反応検査(ツ反)とインターフェロン-γ遊離試験(Interferon-gamma release assay:IGRA)の2つの方法があります。ツベルクリン反応検査は、結核菌(BCG)ワクチン接種を受けたことがあると偽陽性になる可能性があります4)。
IGRA検査は、血液から結核菌特異的エフェクターT細胞を測定することで検査を行います。IGRA検査は、血液検査により行われBCGワクチン接種の有無に左右されません5),6)。
T-スポット.TBの特長
- 感度97.5%、特異度99.1%(国内臨床試験)5)
- 免疫抑制状態の影響を受けにくい 9),13)
- 判定不可が少なく、再検査が少ない12)
T-スポット.TB 検査には、結果に影響を及ぼす可能性のある要因を軽減するための3つのステップがあります7)。これらのステップは、検査の前処理工程のPBMC分離、洗浄、および細胞数測定があります。T-スポット.TB検査を使用することで、信頼性が高まり、結核菌感染検査をより適切に実施できるようになります。
具体的な方法は以下の通りです:
- T-スポット.TB検査では、採血管は1本のみです。
- 全血から末梢血単核球(PBMC)を分離し、洗浄、細胞数を計測します。
- 細胞分離:全血から末梢血単核球(PBMC)を分離
- 細胞洗浄:全血から干渉物質を除去
- 細胞数測定(標準化):細胞数の個人差の影響を低減させるため、分離したPBMCを一定数に揃える
- 結果を目視で確認することができます。
Expert opinions
For World TB Day this year, we asked our experts: 'what's the most impactful action we can take today to accelerate the end of TB?'

"To accelerate the end of TB, we must focus on immediate case identification and treatment, robust contact screening, enhance detection of latent tuberculosis infection through specific advanced diagnostic tools. especially in high-risk and immunocompromised populations. With this multi-prong approach, we can break the cycle of TB transmission and move closer to global TB elimination"
Juzar Ali
MD, Professor (Emeritus) of Medicine at Louisiana State University Health Sciences Center

"The biggest challenge is convincing healthcare providers of the necessity of testing. Especially in patients with HIV or under immunosuppressive therapy, who are at high risk of developing active TB, early detection is key: If you can diagnose and treat LTBI before progressing into active disease, it's much easier, especially if you're comparing it to treating extensively drug-resistant tuberculosis. Early detection and intervention are key to preventing active TB disease and ultimately accelerate the end of TB."
Dr Martin Obermeier
Lab Manager at the Medical Centre for Infectious Diseases in Berlin (MIB)

"TB is widespread in our country, affecting both urban and rural areas. Early detection of all forms of TB is crucial for controlling its spread. Identifying latent TB is essential, as many are unaware, they have it. Diagnosing extra-pulmonary TB, often missed by conventional methods, is critical. We've seen many unexpected cases linked to it. Comorbidities like diabetes or HIV complicate TB cases and require immediate identification. This World TB Day, I hope decision-makers will prioritize early detection and broader use of diagnostic tools to help end TB."
Dr Juliani Dewi, SpPK
Clinical Pathologist Lab Director, Rampal Diagnostika, Indonesia
Featured webinars
Patient stories
Behind every tuberculosis statistic is a human story. The fight against TB is not just about numbers; it's about people - their struggles, resilience, and triumphs.

Kelly
Kelly is a physician and has treated patients with TB. Being a TB patient, however, was new territory for him. One of the most difficult parts of the process was the impact on his family, friends and patients, who were forced to endure months of treatment due to their contact with him. He now brings a new understanding to his practice and works tirelessly to educate others, especially those in the healthcare community.

Taylor
Taylor is a community health worker. Originally from the Philippines, Taylor had latent tuberculosis (LTBI) and then contracted HIV. This caused her LTBI to become active, and she became very sick. After undergoing many months of treatment, Taylor recovered from TB and now works to educate others, particularly those in the LGBTQ community.

Kahyr
Khayr is a North Dakota resident and a successful hotel manager. He spent time living in Somalia as a college student, where he likely contracted multidrug-resistant (MDR) TB. Khayr was told that his chance of survival was 50%. After a long battle, he recovered and now works with We are TB, the survivor advocacy organization.
Featured products


Explore our TB Management related solutions
Blogs
References
- World Health Organization. The End TB Strategy. Geneva; 2014. https://www.who.int/teams/global-tuberculosisprogramme/theend-tb-strategy. Accessed: 2-AUG-23
- Global tuberculosis report 2024. Geneva: World Health Organization; 2023. Licence: CC BY-NC-SA 3.0 IGO.
- World Health Organization. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management.
- Schluger NW, Burzynski J. Recent advances in testing for latentTB.Chest. 2010 Dec; 138(6): 1456–63.
- T-スポット.TB 添付文書(第10版)
- QuantiFERON®-TB Gold Plus ELISA Kit Instructions for Use. L1123669_R3_IVDr_QF_ELISA_ROW_0323_FINAL. March 2023.
- World Health Organization. WHO operational handbook on tuberculosis. October 1, 2022.
- Banaei N, Gaur RL, Pai M. Interferon gamma release assays for latent tuberculosis: what are the sources of variability? Journal of clinical microbiology. 2016; 54(4): 845–850.
- Wong SH, Gao Q, Tsoi KK, Wu WK, Tam LS, Lee N, Chan FK, Wu JC, Sung JJ, Ng SC. Effect of immunosuppressive therapy on interferon γ release assay for latent tuberculosis screening in patients with autoimmune diseases: a systematic review and meta-analysis. Thorax. 2016 Jan.
- Bèlard, et al. Prednisolone treatment affects the performance of the QuantiFERON gold in-tube test and the tuberculin skin test in patients with autoimmune disorders screened for latent tuberculosis infection. Inflammatory Bowel Diseases, Volume 17, Issue 11, 1 November 2011, Pages 2340–2349.
- Komiya K, Ariga H, Nagai H, et al. Impact of Peripheral Lymphocyte Count on the Sensitivity of 2 IFN-γ Release Assays, QFT-G and ELISPOT, in Patients with Pulmonary Tuberculosis. Intern Med. 2010; 49(17): 1849–55.
- Rego K, et al. Utility of the T-SPOT®.TB test’s borderline category to increase test resolution for results around the cut-off point. Tuberculosis. 2018; 108:178 185.doi:10.1016/j.tube.2017.12.005.
- Clark SA, Martin SL, Pozniak A, et al. Tuberculosis antigenspecific immune responses can be detected using enzymelinked immunospot technology in human immunodeficiency virus (HIV)-1 patients with advanced disease. Clin Exp Immunol. 2007; 150(2):238–244.
本製品は体外診断用です。認可された地域でのみご利用いただけます。製品の提供状況については、お近くの担当者までお問い合わせください。Revvity Inc.は、研究、医薬品、または治療法に関する推奨や支持を行うものではありません。本ページ情報提供を目的としたものであり、医学的アドバイスを意図したものではありません。