
Explore our solutions
Biologics immunogenicity and PK/PD
The immunogenic potential of biotherapeutic drug molecules can have an effect at any point, from its administration through to metabolism and elimination. Therefore, it is important to understand the impact of immunogenicity upon the PK and PD properties of a large molecule drug.
The characterization of the effect of anti-drug antibodies on the PK is critical. The development of a physiologically relevant PK model in the context of the immunogenicity profile offers significant value to preclinical development.
We offer technology platforms that enable in vitro, ex vivo, and in vivo evaluations to help you understand the immunogenicity of your biologics.


マイクロ流体核酸分析
従来のゲル分離を簡略化し、わずかな時間でデータの再現性を向上させることができます。LabChip™自動マイクロ流体キャピラリー電気泳動技術を利用した分離では、ゲノムサンプルを数十秒で分析できます。

In Vivo Imaging Instruments
Whether you’re trying to understand biology pathways, monitor disease progression, or evaluate drug candidates earlier in drug development, our wide portfolio of preclinical imaging systems are designed to meet your research needs.

ハイコンテントスクリーニング装置
ハイコンテント解析(HCA)またはハイコンテントスクリーニング(HCS)は、ハイスループットの自動イメージングと解析を組み合わせ、シングルセルレベルで定量的なマルチパラメトリックデータを抽出します。
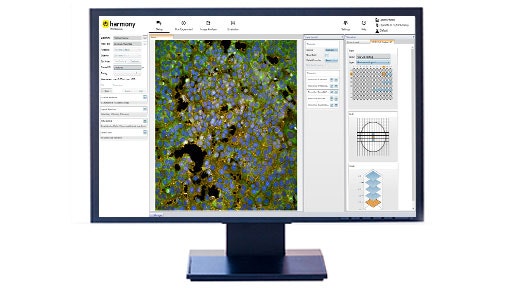
Cellular Imaging Software
Revvityは、より効果的かつ効率的に画像データを取得、可視化、解析、共有するための洗練された細胞イメージングおよび画像解析ソフトウェアを提供しています。
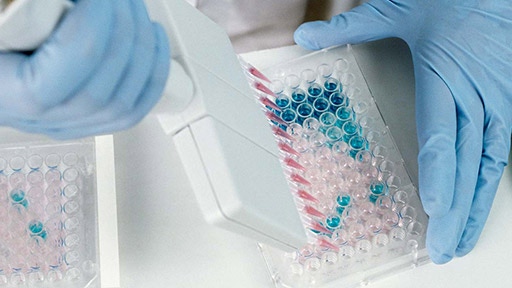
In vitro assay
Leverage reliable in vitro assays to help better understand the interactions of the innate and adaptive immune response for your discovery and development of immunotherapies
We offer a range of assays for characterizing cytokine response, screening biologics, measuring signalling events, determining mechanism of action, and understanding the antibody-dependent cellular cytotoxicity (ADCC) of the biologic.

Luminescence Assays
Luciferase assays allow for the study of transcriptional gene expression, virus life cycles, and cell viability.

放射性物質の検出
放射性物質検出は、薬物経路の分析、研究室の環境保護、環境汚染の評価、食品の安全性のモニタリングなど、多くの科学的用途で研究者に使用されてきたゴールドスタンダードであり、現在もその地位を維持しています。

Radionuclides
Radionuclides provide a highly specific and sensitive labeling option for proteins, cells, and tissues.

Immune Cell Screening
Preclinical screening services using primary human immune cells to address biologically relevant questions for target identification and lead optimization































