-
cAMP kits

The large majority of receptors of interest for second-generation obesity drug discovery & development are Gs-coupled receptor, which makes cAMP quantification the most relevant and prevalent method of screening and characterization. Revvity carries a range of cAMP assays for all applications, including specific Gs assay adapted to the pharmacology of these GPCRs:
- HTRF cAMP Gs Dynamic for increased sensitivity
- HTRF cAMP Gs HiRange for wider assay windows
- HTRF cAMP Gi
- LANCE Ultra cAMP
-
IP-One kits

Many Gs-coupled receptor, including GLP-1R & GIPR, have secondary Gq coupling that accounts for subtle variation between ligands. Adding that layer of characterization to drug candidates is relevant to the fine-tuning that this new generation of molecules calls for.
Fontaine, T., Busch, A., Laeremans, T. et al. Structure elucidation of a human melanocortin-4 receptor specific orthosteric nanobody agonist. Nat Commun 15, 7029 (2024).
- HTRF IP-One
- AlphaLISA IP-One for increased sensitivity & wider assay window
-
Beta-arrestins Kits

Beta-arrestin recruitment & receptor internalization appears to be an important characteristic of successful molecules so far, with scientists suggesting lignad bias is key to the design of fine-tuned drug candidates. For instance, the dual GLP-1R/GIPR agonist Tirzepatide shows native ligand-like signaling at GIPR in terms of G-protein signaling and beta-arrestin recruitment, but exhibits a strong bias toward G-protein signaling at GLP-1R, where the recruitment of arrestin and receptor internalization are much less promoted. Revvity offers several beta-arrestin assays, including a beta-arrestin recruitment solution designed to study ligand bias in endogeneous beta-arrestin expression level settings.
-
Tag-lite binding assay kits

Tag-lite is a non-radioactive, cell-based technology that enables the investigation of natural ligands, small molecules or antibodies binding to cell surface receptors. It is especially advantageous for GPCR and, as well as for the study of real-time ligand kinetics.
SNAP-tag receptor expressing cell line
Ready to use binding assay reagents
- GLP1 receptor Frozen & Labeled Cells
- GIP receptor Frozen & Labeled Cells
- GLP1 receptor Red Agonist Ligand
- GIP receptor Red Agonist Ligand
Transfect your cells of choice with SNAP-tagged receptors
-
Phospho assay kits

The transduction of Gi and Gs-coupled Receptor-dependent downstream signals is operated by phosphorylation cascades under the regulation of cAMP levels via PKA activation/inhibition. These cascades are varied and branch out into an array of pathways that depends on the receptor initiating the signal, cell types, tissues, etc. Revvity offers a rich portfolio of cell signaling assays with over 150 analytes to study complex signaling pathways at every step of their phosphorylation cascade. Among these, a few phosphoproteins are consistently involved in the early stages of GPCR signaling and add a layer of characterization when designing finely-tuned ligands candidates fro Obesity treatment.
- ERK1/2 Detection Kits (Phospho Thr202/Tyr204 & Total)
- CREB Detection Kits (Phospho Ser133 & Total)
- CC-RAF Detection Kits (Phospho Ser338 & Total)
Obesity
Power up your peptide therapeutics and small molecule discovery for obesity with a complete portfolio of GPCR assays
As of 2025, the success of GLP1R and GIPR agonists like Semaglutide (Novo Nordisk) and Tirzepatide (Eli Lilly) has sparked massive interest in the development of more peptide therapeutics with dual or triple agonism for GLP1R, GIPR, and GCGR, while also opening up the possibility of investigating other receptor families. Revvity aims to provide researchers with a full suite of GPCR reagents and kits that enable access to all the binding, pharmacology, and pharmacodynamics characteristics of GPCR ligands, allowing the fine-tuning of these multi-agonistic compounds.
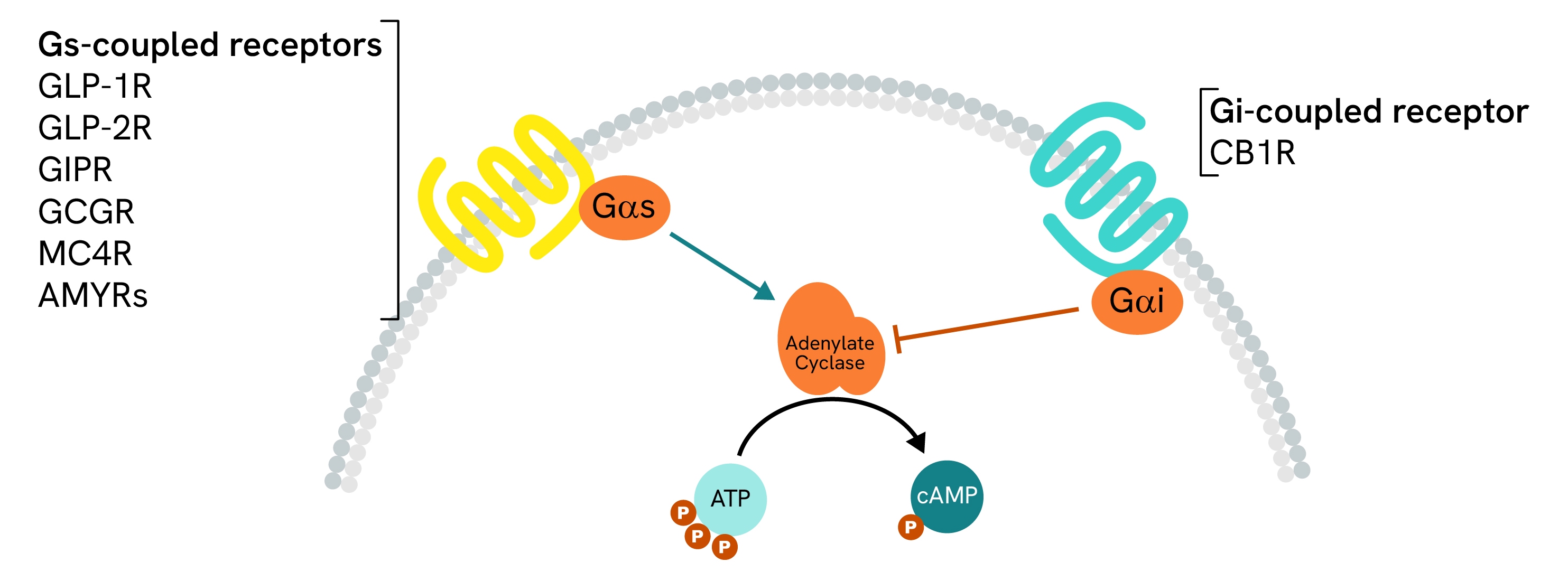
Gs-coupled receptor activity with specialized cAMP assays
The large majority of receptors of interest for obesity therapeutics have a primary coupling that can be assessed using our dedicated HTRF cAMP Gs assays. Our assays are available in both Gs and Gi formats for accurate measurement of either type of GPCR and are a tool of choice for the discovery and characterization of obesity and GLP1-related compounds, as presented in recent literature on the topic.
Learn how our cAMP Gs assays are used to identify and characterize GLP1R and GIPR agonists:
- Characterization of GLP1R agonists and their effects on insulin levels here
- GLP1R agonists / HTRF cAMP Gs dynamics here
- Triple agonist / HTRF cAMP Gs dynamics here
- Human and mouse GLP1R and GIPR / HTRF cAMP Gs dynamics here
Keep an eye out for secondary Gq-coupling with IP-One
Add an IP-One readout to account for the secondary Gq-coupling exhibited by most primarily Gs-coupled receptors, including GLP1R, GIPR, and MC4R.
Learn how our IP-One assay was used alongside cAMP detection to account for Gq-secondary coupling of MC4R in the characterization of agonist compounds in this paper.
Beta-arrestin recruitment
Beta-arrestin recruitment and beta-arrestin-dependent internalization are key characteristics of the successful Tirzepatide drug. For this reason, assessing beta-arrestin recruitment induced by potential drug candidates is a staple of anti-obesity research and is featured abundantly in the literature. The HTRF beta-arrestin 2 recruitment assay enables the characterization of biased signaling of compounds in endogenous settings, providing insights into the true pharmacology of compounds for accurate characterization.
Real-time fluorescent receptor binding assays with Tag-lite
Tag-lite enables the assessment of real-time compound binding profiles in a non-radioactive format. This non-radioactive, cell-based technology enables the investigation of natural ligands, small molecules, or antibodies binding to cell-surface receptors. It is especially advantageous for studying GPCR and real-time ligand kinetics.
Learn how Tag-lite is used to monitor GLP1R binding and internalization:
- Tag-lite Binding Assay with GLP1R in this paper
- Tag-lite Internalization of GLP1R in this paper
Diabetes
Revvity provides easy-to-use no-wash reagents and detection kits for diabetes research. Diabetes is a complex, heterogeneous condition affecting over 400 million people worldwide, with serious consequences for multiple organs and metabolic pathways.
Our portfolio of assays is organized around the five key organs involved in or affected by Type 2 diabetes. Each reagent and kit is designed to map specific cell-signaling cascades related to metabolic function in:
- Muscle cells
- Adipocytes
- Liver hepatocytes
- Pancreatic α and β cells
- Intestinal L-cells
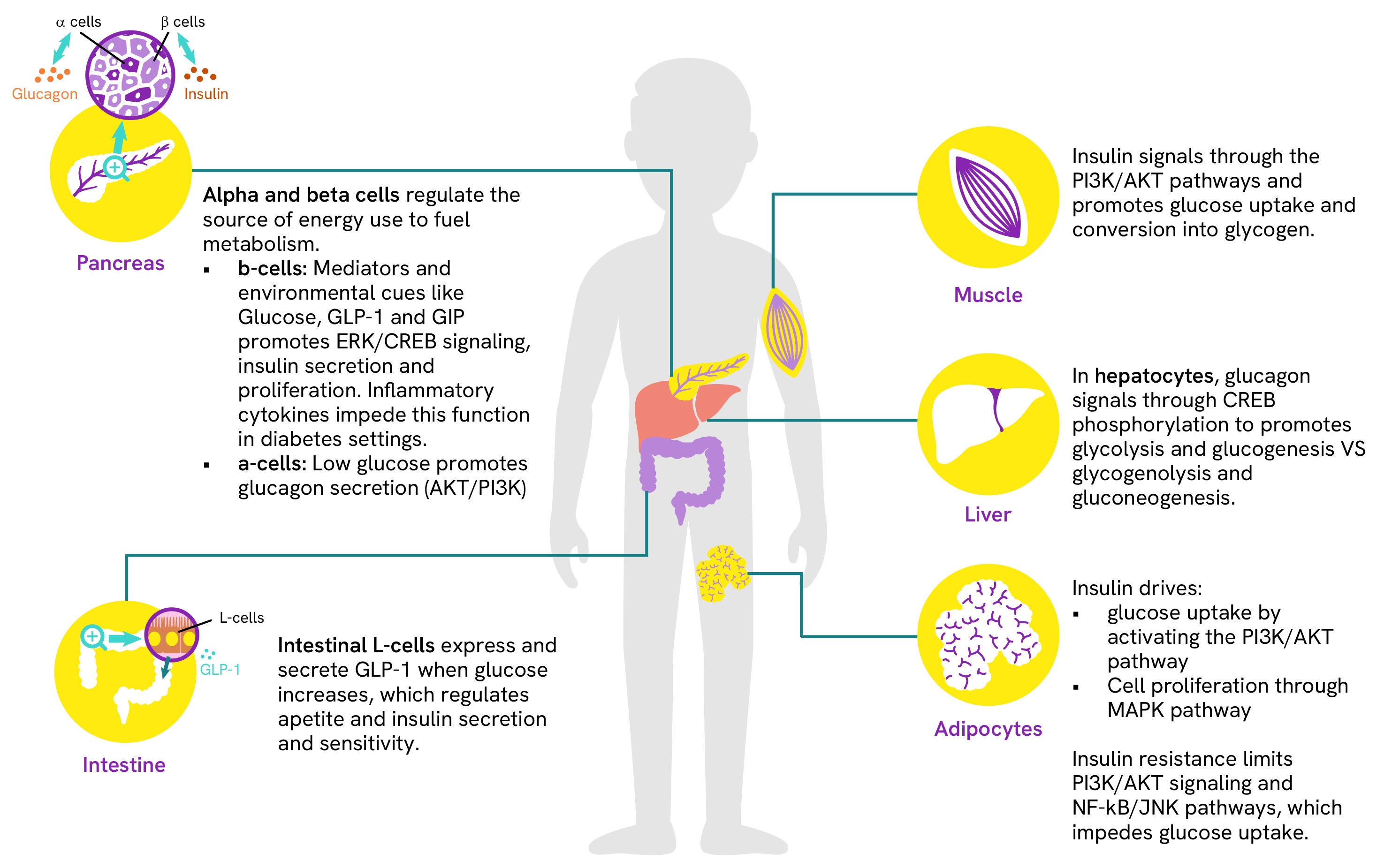
Simple and robust assays for measuring glucose and insulin signaling in pancreatic β-cells
β-cells play a crucial role in diabetes as they are responsible for insulin secretion. These specialized cells employ two primary signaling cascades for their normal function. The first responds to glucose uptake and signals via intracellular calcium levels and cAMP, triggering insulin secretion and the expression of IRS1/2. The second pathway involves insulin-induced autoactivation, promoting cell differentiation, survival, and protein synthesis. This dual functionality makes β-cells central to diabetes, as their insulin secretion and sensitivity are interdependent, regulating glucose uptake and metabolism throughout the body. The study of β-cell activity therefore requires robust and accurate measurement of signaling proteins at key points within these cascades to investigate signal transduction and assess the effectiveness of drug candidates.
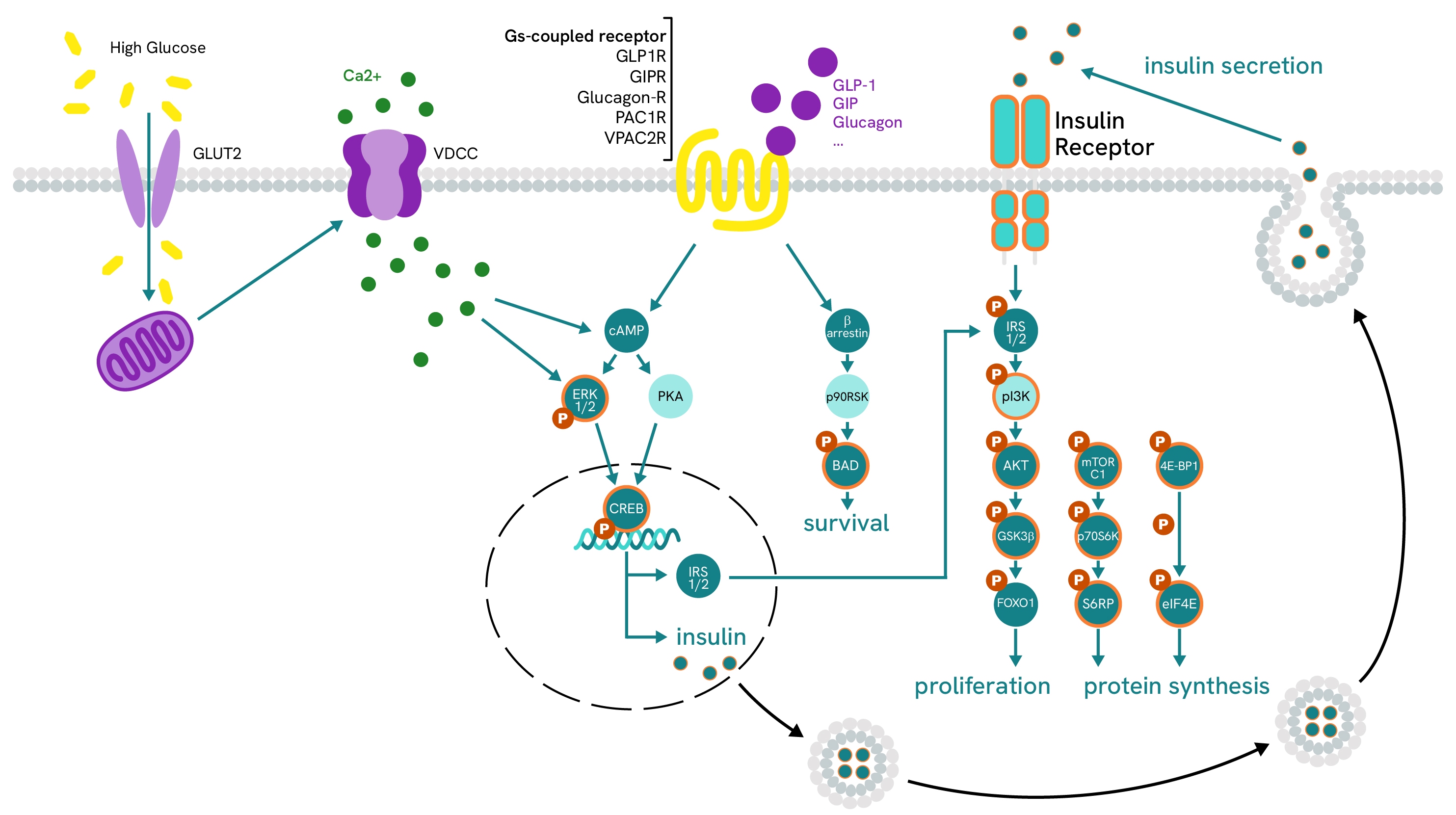
Learn more about the detailed pathways for all five organs and cell types involved in diabetes in our dedicated guide.
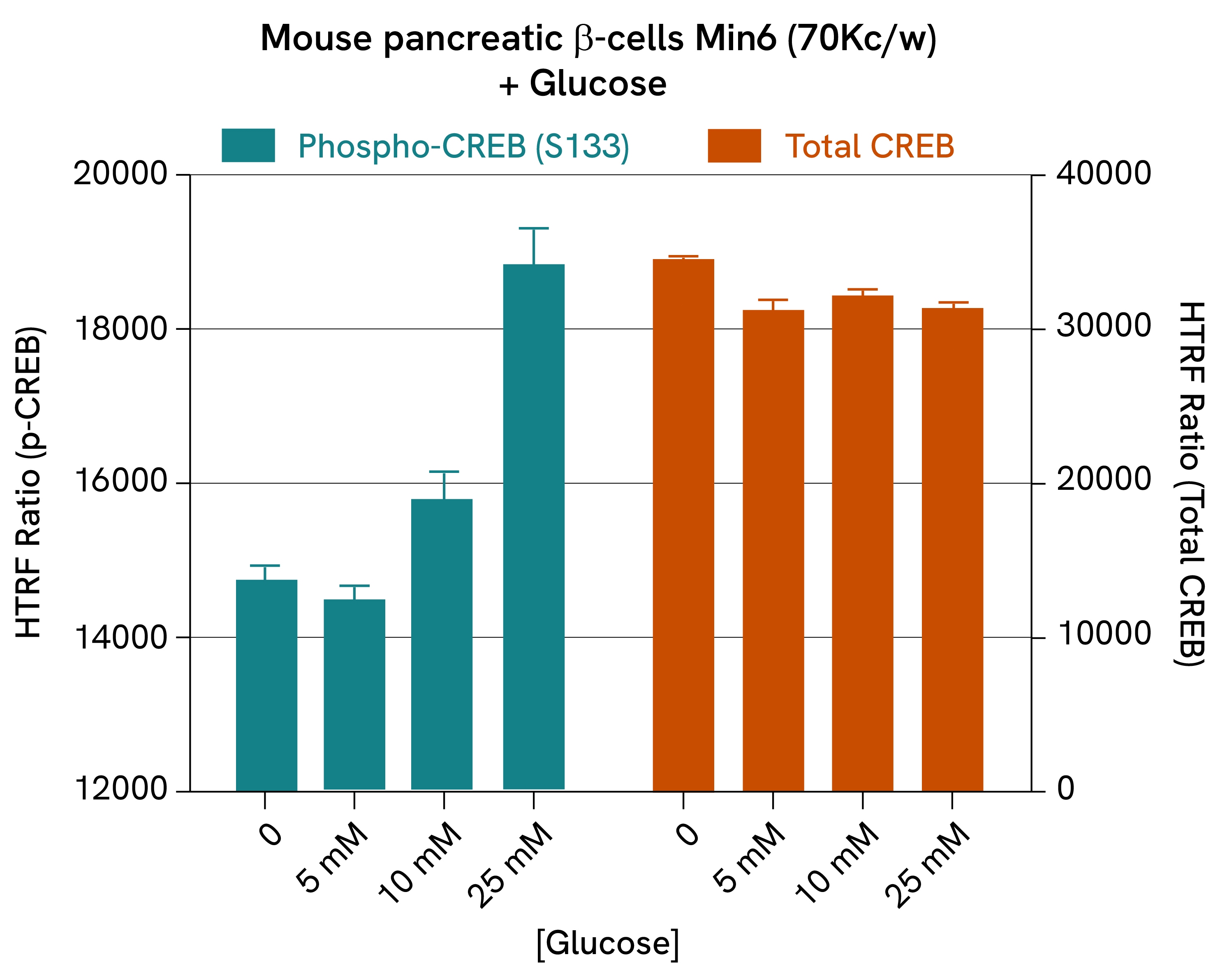
Glucose-induced CREB phosphorylation:
The phosphorylation of transcription factor CREB is performed by cAMP-dependent pathways and is linked to multiple signaling cascades. In β-cells, it is promoted by high glucose levels and Gs-coupled receptor signaling (such as GLP1R and GIPR) and is a driver of insulin expression.
In this study, mouse pancreatic ß-cells (Min6) were stimulated with KRB and increasing concentrations of glucose for 5 minutes at 37°C. After a 30-minute lysis incubation time, total and phospho-CREB levels were measured. The results revealed a dose-dependent increase in CREB phosphorylation, while total protein levels remained stable.
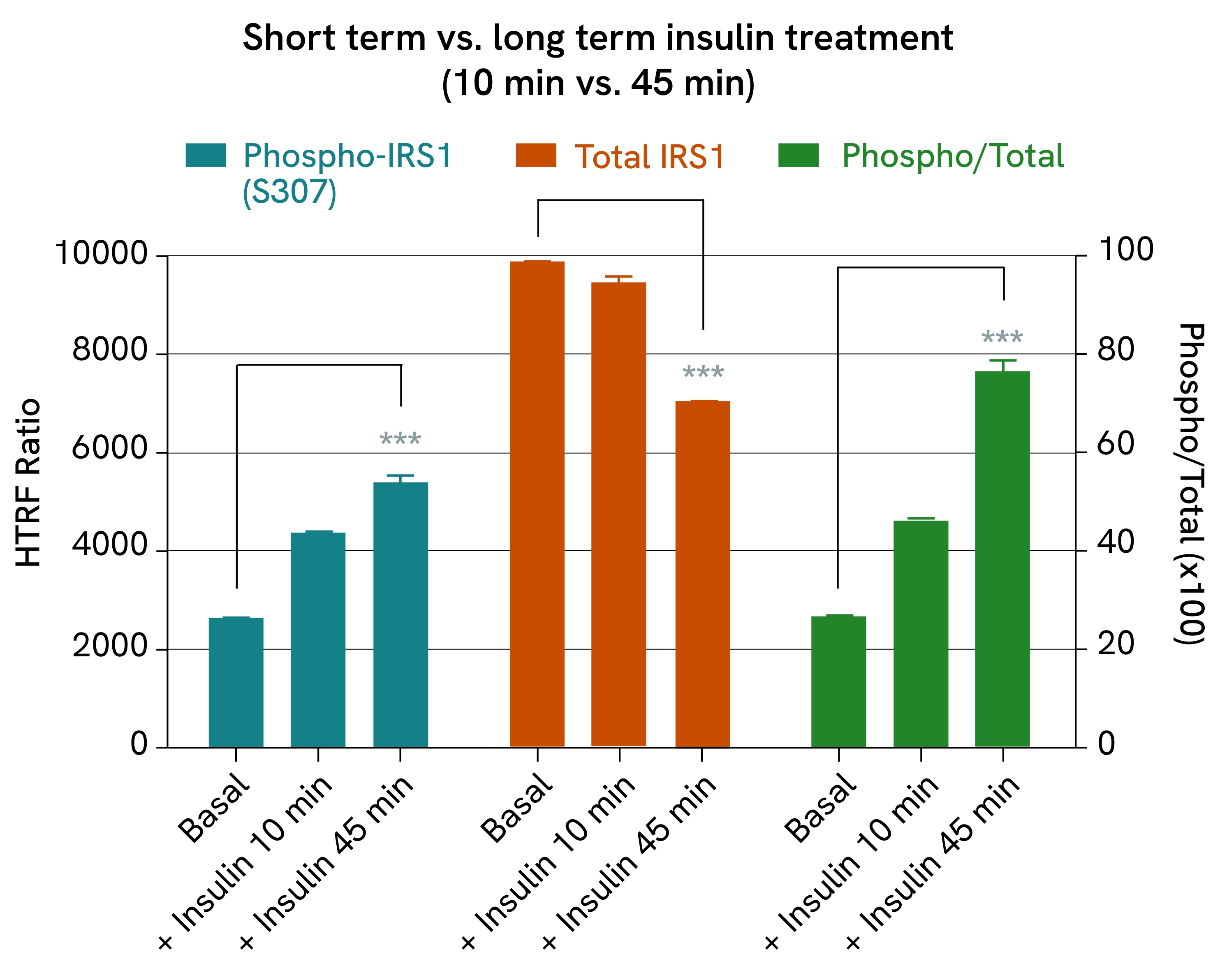
Insulin-induced IRS1 phosphorylation:
IRS1/2 expression is a key component of glucose-signaling in β-cells, where it allows auto-induced insulin activation and promotes signaling pathways involved in proliferation and protein synthesis. In other metabolic organs, such as muscle, IRS1/2 also regulates glucose uptake by triggering the internalization of glucose transporters after a glucose peak.
Here, we demonstrate an example of IRS1 phosphorylation in C2C12 cells (mouse muscle cells) treated with insulin for 10 or 45 minutes. After removing the cell supernatant, the cells were lysed and IRS1 phospho- and total forms were quantified using the corresponding HTRF kits. The results showed that IRS1 phosphorylation increased over time, which is consistent with prolonged insulin-induced signaling. Although the total amount of IRS1 remained constant after 10 minutes, it decreased after 45 minutes, which is in line with a retrocontrol loop that reduces the efficiency of prolonged insulin signaling over time.
Insulin
Under normal circumstances, insulin binds to its receptor (IR), initiating a signaling cascade that translocates the GLUT4 receptor from cytosolic vesicles to the membrane. Here, it binds glucose, increasing glucose absorption into the affected cells. In addition to promoting glucose absorption, insulin promotes its degradation through glycolysis and inhibits gluconeogenesis in the liver. It also stimulates the assimilation of excess sugars into lipids by lipogenesis while inhibiting lipolysis in fatty tissues. Overall, insulin shifts metabolism toward the use of available sugars as energy sources instead of fats.
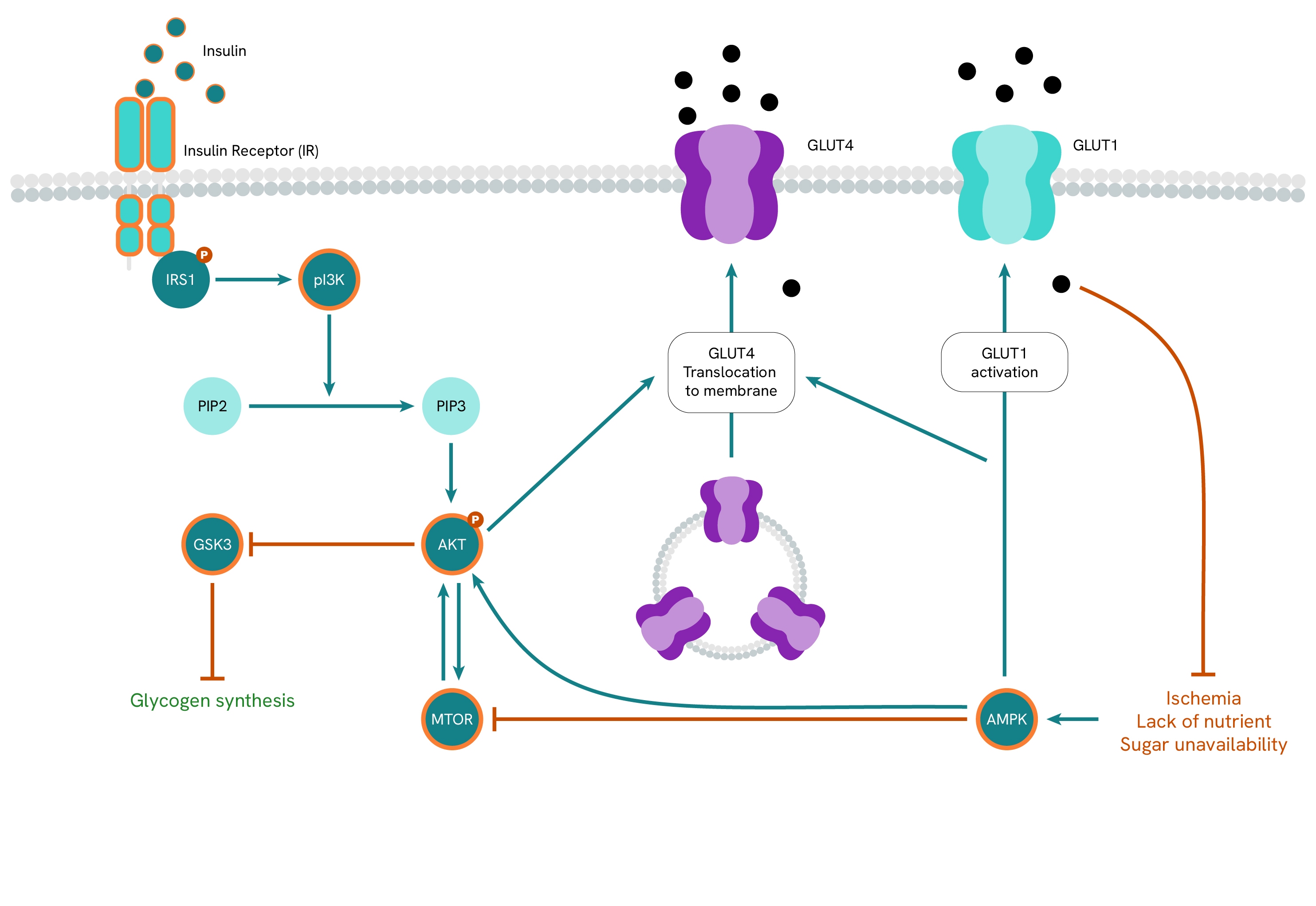
Insulin resistance impairs these regulatory functions, as cells become less sensitive to insulin and require higher levels of the hormone to achieve the same results. To compensate for this, the body resorts to producing increased levels of insulin, leading to hyperinsulinemia. However, this course of action is not a long-term solution, as it not only carries consequences for the many processes regulated by insulin, but it also fails to correct insufficient glucose absorption, resulting in chronic hyperglycemia.
Learn more about the role of insulin in metabolism and the signaling pathways involved in insulin resistance in our guide dedicated to obesity.
Robust and precise insulin assays for all applications
Accurate quantification of insulin in various sample matrices is essential in preclinical studies of insulin secretion. Revvity offers a comprehensive range of insulin quantification assays designed to significantly improve performance over traditional ELISA methods in terms of:
- Reduced time to results
- Low sample consumption
- Cost-effectiveness of quantifying insulin
All Revvity assays have been extensively validated on relevant cellular models for insulin release and show high correlation with established methods.
High range kit
Highly concentrated samples often need to be diluted prior to insulin quantification, which can lead to inaccuracies and increase time to results. With no washing steps prior to insulin quantification and a 4-log dynamic range, the HTRF Insulin High Range assay kit delivers the benefits of fewer sample dilutions and increased accuracy, ensuring straightforward and reliable quantification of insulin in highly concentrated samples.
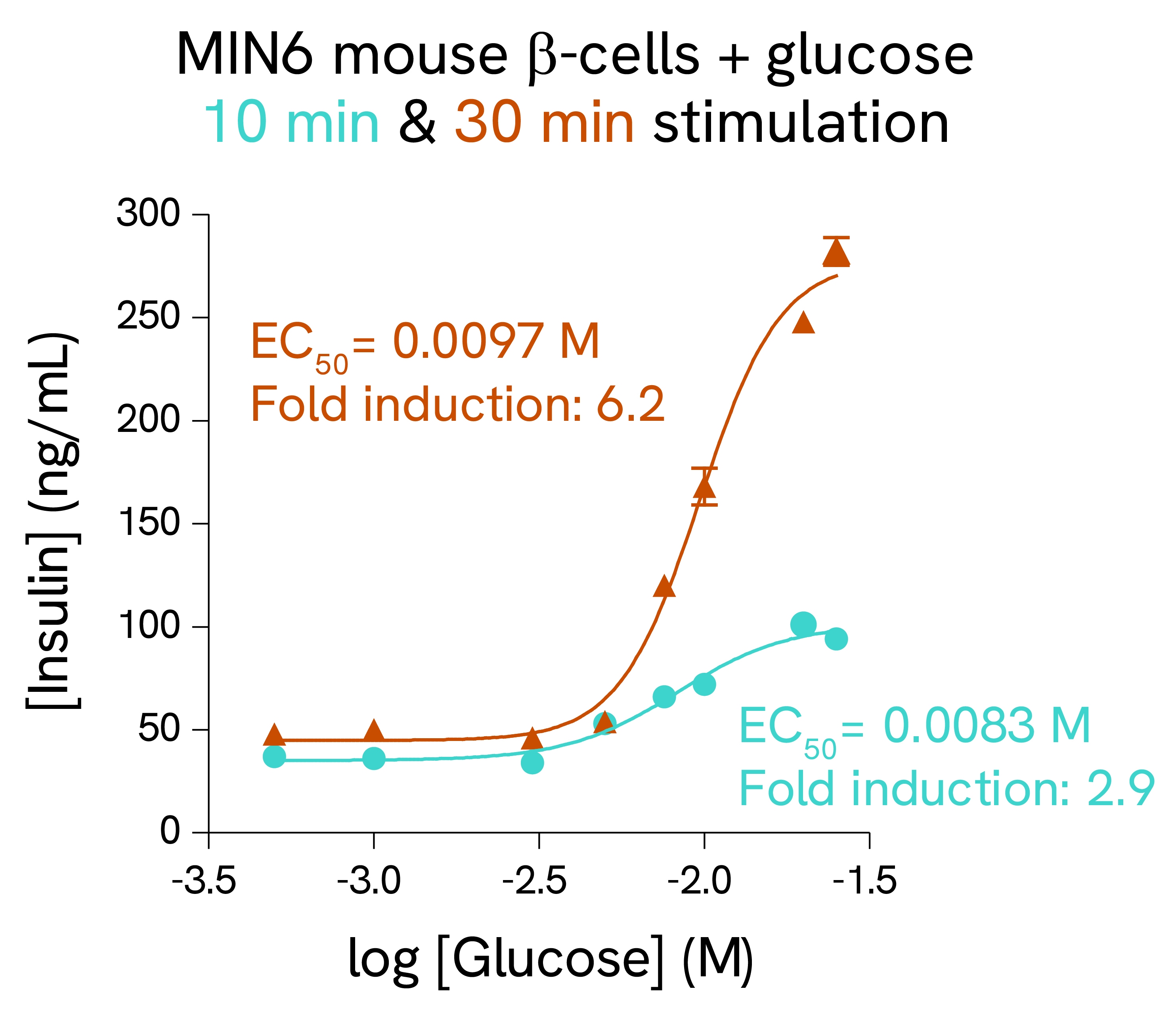
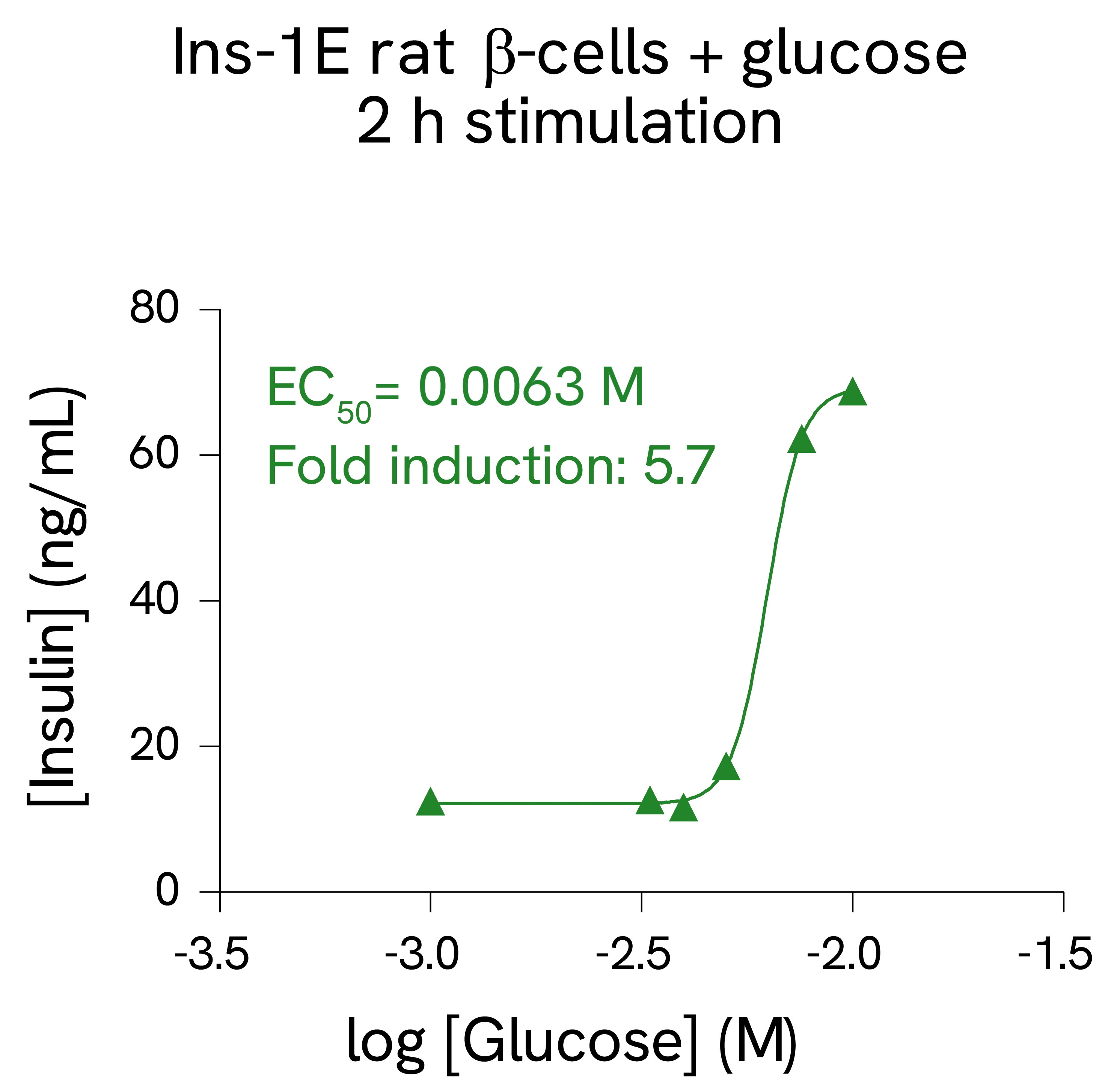
Validation on rat and mouse β-cells:
MIN6 mouse β-cells and INS-1E rat β-cells were stimulated with increasing concentrations of glucose. After 10 and 30 minutes secretion for MIN6 cells, or 2 hours secretion for INS-1E cells, cell supernatants were collected, and insulin concentration was measured using HTRF detection reagents. Each treatment condition was measured in triplicate (error bars represent SEM).
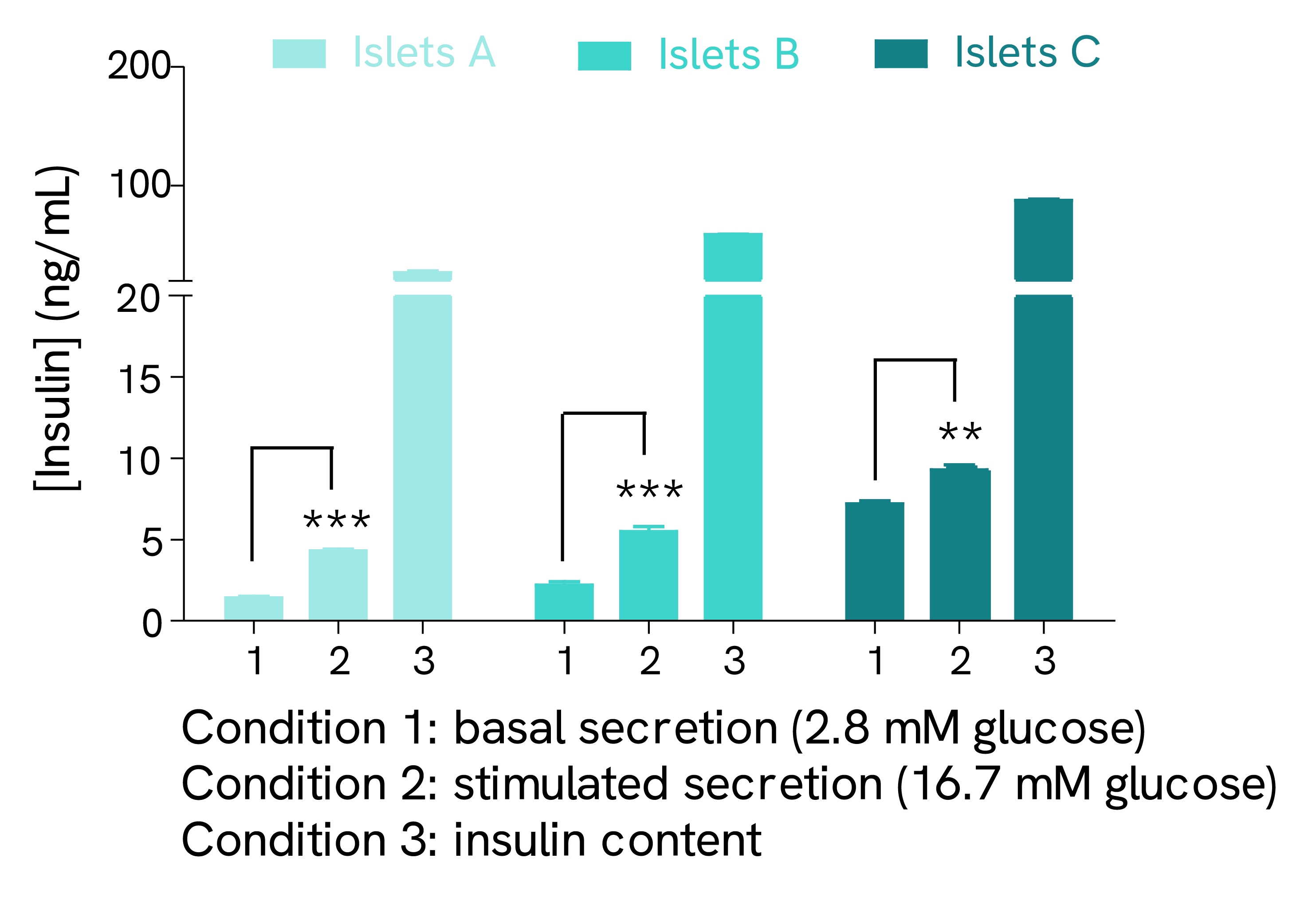
Validation on human pancreatic islets
Pancreatic islets isolated from three human pancreases (A, B, and C) were pre-incubated with 2.8 mM glucose. The islets were then incubated in the same solution for 1 hour at 37°C and the supernatant was collected to measure basal insulin secretion. The same islets were stimulated for 1 hour at 37°C with 16.7 mM glucose before collecting the supernatant. At the end of the secretion test, the islets were lysed, and the lysate was collected and centrifuged. The insulin concentration of supernatants (stimulated secretion) and lysates (insulin content) was determined using the Insulin High Range kit reagents.
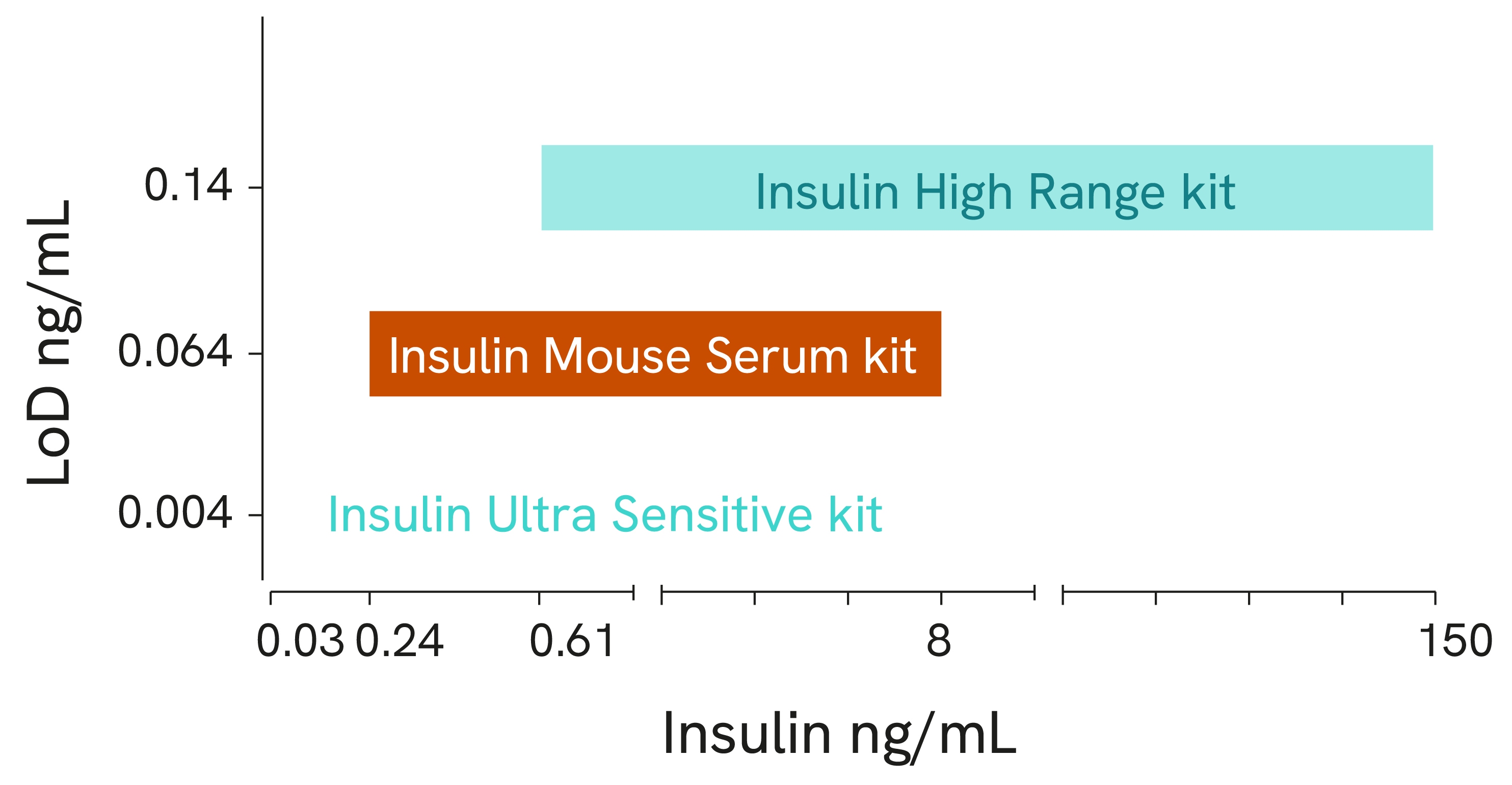
Ultra Sensitive Kit
One hurdle faced by researchers looking to quantify insulin is the high number of islets often required per assay. High assay sensitivity can significantly reduce the number of islets needed per well. With a sensitivity of around 4 pg/mL, the HTRF Insulin Ultra Sensitive assay is designed with precious sample economy in mind, allowing for a significant reduction in the number of islets required per test. The enhanced sensitivity also enables the quantification of basal insulin levels from cells and insulin released from perfused mouse pancreas models, which typically generate low insulin levels.
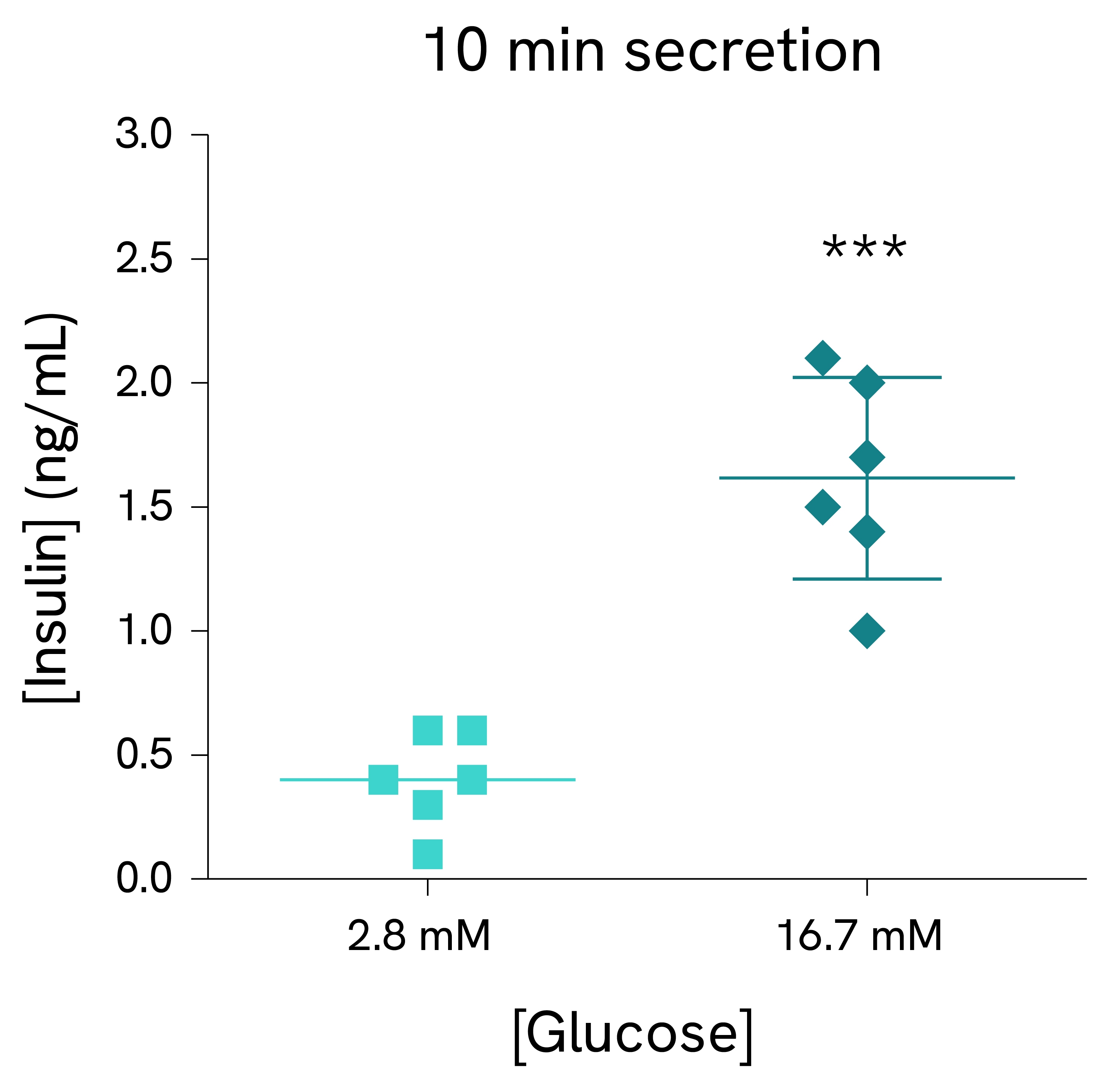
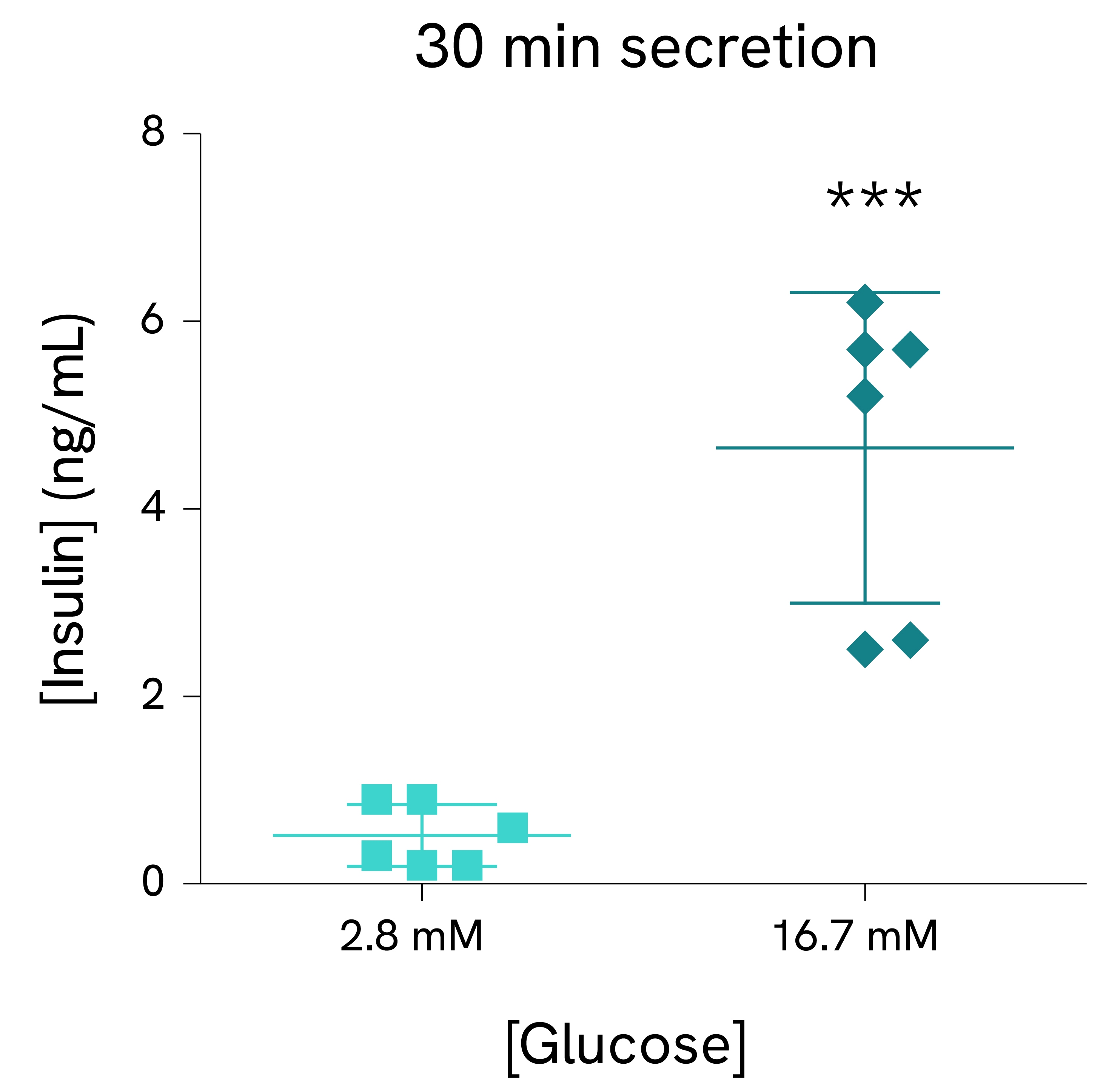
Validation on mouse pancreatic islets:
Pancreatic islets were isolated from C57BL/6N male mice and dispensed in tubes with KRB buffer containing 2.8 mM or 16.7 mM glucose (5 islets/tube, 6 tubes/condition). After 10 minutes (left) or 30 minutes (right) of incubation, supernatants were collected, and the insulin concentration was measured using the HTRF Insulin Ultra Sensitive assay kit reagents.
The measurement of insulin using Revvity’s HTRF platform offers significant advantages over traditional ELISA technology. HTRF provides the specificity, sensitivity, and accuracy of an ELISA, but at a fraction of the time and with reduced sample volume. Designed for low sample consumption, the Insulin Mouse Serum kit is ideal for insulin quantification in precious serum samples. The Insulin High Range kit offers an extended dynamic range, circumventing the need for dilution in highly concentrated samples. Finally, the Insulin Ultra Sensitive kit detects even the lowest concentration of insulin in hard-to-measure samples.

See all details and validation data for HTRF Insulin Assays in various samples and cell types in our dedicated guide.


Featured resources
References
- https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- Jones B,et al. In vivo and in vitro characterization of GL0034, a novel long-acting glucagon-like peptide-1 receptor agonist. Diabetes Obes Metab. 2022 Nov;24(11):2090-2101. doi: 10.1111/dom.14794. Epub 2022 Jul 18. PMID: 35676825; PMCID: PMC9796023.
- Lucey M, et al. Disconnect between signalling potency and in vivo efficacy of pharmacokinetically optimised biased glucagon-like peptide-1 receptor agonists. Mol Metab. 2020 Jul;37:100991. doi: 10.1016/j.molmet.2020.100991. Epub 2020 Apr 8. PMID: 32278079; PMCID: PMC7262448.
- Coskun T et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept. Cell Metabolism, Volume 34, Issue 9, 1234 - 1247.e9. c. doi: j.cmet.2022.07.013
- McGlone E.R et al. Chronic treatment with glucagon-like peptide-1 and glucagon receptor co-agonist causes weight loss-independent improvements in hepatic steatosis in mice with diet-induced obesity. Biomedicine & Pharmacotherapy, Volume 176, 2024. https://doi.org/10.1016/j.biopha.2024.116888
- Fontaine, T., Busch, A., Laeremans, T. et al. Structure elucidation of a human melanocortin-4 receptor specific orthosteric nanobody agonist. Nat Commun 15, 7029 (2024). https://doi.org/10.1038/s41467-024-50827-7
- Chichura, K.S., Elfers, C.T., Salameh, T.S. et al. A peptide triple agonist of GLP-1, neuropeptide Y1, and neuropeptide Y2 receptors promotes glycemic control and weight loss. Sci Rep 13, 9554 (2023). https://doi.org/10.1038/s41598-023-36178-1
- Roed SN, et al. Real-time trafficking and signaling of the glucagon-like peptide-1 receptor. Mol Cell Endocrinol. 2014 Feb 15;382(2):938-49. doi: 10.1016/j.mce.2013.11.010. Epub 2013 Nov 22. PMID: 24275181.





























