Services
Revvity has collaborated with industry and academia to optimize the drug identification process using our powerful and accurate technologies. Beyond our extensive catalog of assays, an experienced Project Manager will evaluate your project’s feasibility and collaborate with you to develop a customized assay for your specific target of interest.
We are committed to supporting your custom assay project with a dedicated team of scientific experts, maintaining strict confidentiality with respect to your project and your target. We will carefully evaluate what’s possible and determine an accurate and time-efficient approach to your objectives. Additionally, we will check the solution by running it on your own equipment.
We offer three custom assay options, providing a range of approaches to meet various needs:
Biochemical assays
We utilize only purified reagents for enzyme assays, protein quantification, or protein-protein interaction assays to elucidate your target’s biology.
ELISA conversions
We can convert any of your ELISA assays into an AlphaLISA™, DELFIA®, or HTRF® format, thus eliminating time-consuming and expensive steps.
Cell-based assays
This customized solution will work with your specific cells. The reagents are carefully selected and optimized on your lysates to ensure consistent, reliable, and high-quality results on your preferred cell model. pment.
Expertise
Customer-focused company with high technology offerings
We offer 30+ years of experience in custom laboratory services. Our experts employ a straightforward assay development process tailored to your specific technology and assay requirements. A key aspect of our custom assay development service is the scientist-to-scientist interaction you receive, from the start of your project to completion. Our aim is to make custom assay development simpler and easier for drug-discovery and development scientists.
Focus on the technology
Our scientists focus on premier technology options designed to accelerate your research, and in doing so, have developed a deep understanding of the mechanisms involved.
Your project will receive the same attention and discerning development techniques as the hundreds of kits Revvity has in its portfolio.
Consistency and persistence
Psychologist K. Anders Ericsson developed the concept of deliberate practice to explain the development of extraordinary abilities across disciplines, including sports and medicine. He attributed the development of these skills to consistency and persistence. We wholeheartedly agree.
Our scientists have extensive experience developing custom assays and facing complex and diverse case scenarios.
Customer-centric approach
Revvity genuinely cares about its customers and their projects. We recruit our scientists based not just on their skills but also on their ability to develop empathy for our clients’ projects.
At Revvity we understand that there is no one solution for the development of your ideal assay and that every project brings its own set of challenges. For that reason, we are organized around regional laboratories and encourage frequent conversations between our scientists and you, the future end users.
Chances of success
Our policy is to always be honest on the chances of success for any project. We bet on rational explanations to build trust with our clients.
This philosophy has resulted in the development of the Revvity Proposal, a document that details the tentative experimental plan and provides a realistic assessment of the chances of success. The Proposal is complimentary.
Deliverables
Why only get a protocol when you can have a custom kit?
All assay development projects result in the delivery of a custom kit which includes a protocol and a fixed quantity of reagents to perform an agreed-upon number of tests.
Development process and delivery
In as little as 4 to 8 weeks, we will develop and deliver your customized assay.
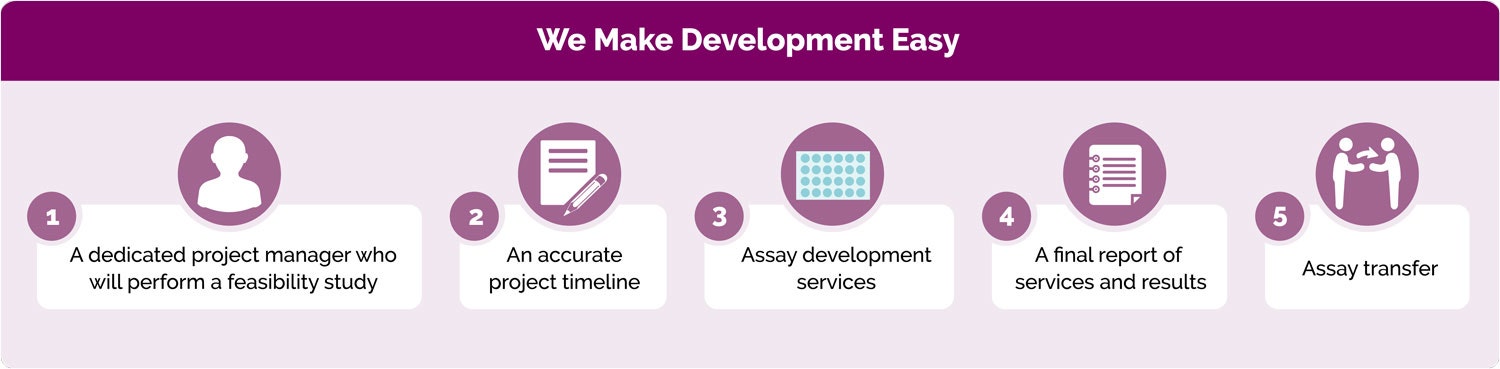
Thanks to our extensive experience, Revvity’s customized assay process is both flexible and fast. We begin by assigning you a dedicated Project Manager who will work diligently to align each aspect of the process with your requirements and timeline, while respecting the level of NDA and MTA confidentiality you specify. The Project Manager will be available to answer any questions throughout the project, as well as to update you on the progress being made towards delivering your customized solution.
First, we will deliver a feasibility study with complete assay specifications, including the options available to you, reagent description, and a precise development timeline. Then, the 4 to-8 week development process begins. Leveraging our depth of experience, we’ll work quickly to deliver your customized solution. We can modify our direction of study easily to accommodate any input you may provide during the project. Your assay will be optimized as we select and label reagents.
Finally, we’ll work with you on assay transfer to your own laboratory. Additionally, we can also help you with assay implementation and final report development.