CHOSOURCE ADCC+ cell line
Increase biotherapeutic potency and efficacy with CHOSOURCE ADDC+ cell line
CHOSOURCE™ ADCC+ cell line eliminates the cell’s natural fucosylation activity which can increase therapeutic protein efficacy and potency. Elimination of core fucose on the glycan of the Fc-region of antibodies enhances antibody-dependent cellular cytotoxicity (ADCC) activity of immune effector cells by increasing antibody binding to the CD16a receptor present on effector cells.
CHOSOURCE ADCC+ cell line: How can it help?
This cell line is a double knockout in both the glutamine synthetase (GS) and fucosyltransferase genes. This unique modification enables the production of completely afucosylated proteins within a traditional GS background.
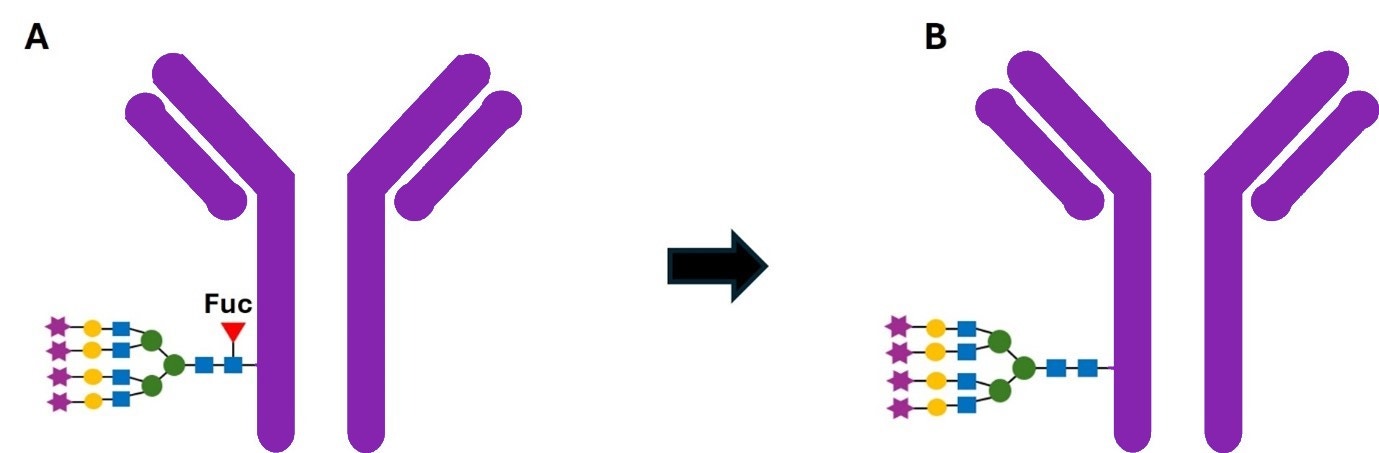
Figure 1: A) Fucosylated antibody (with the fucose residue denoted by ) and B) Afucosylated antibody.
ADCC is a mechanism of immune defense where an effector cell of the immune system kills a target cell whose surface antigens have been bound by specific antibodies. Effector cells such as natural killer (NK) cells interact with antibodies through the antibody-binding FcγRIIIa receptor, this binding is essential for the release of cytotoxic factors that cause the death of the target cell.
The use of afucosylated antibodies increases the binding between the Fc region of the antibody and the receptor enhancing the therapeutic efficacy of antibodies, particularly in oncology, anti-infectious and anti-inflammatory conditions. This may result in an increase in drug potency, expanding therapeutic window and reducing dosage requirements thereby potentially reducing undesirable side effects.
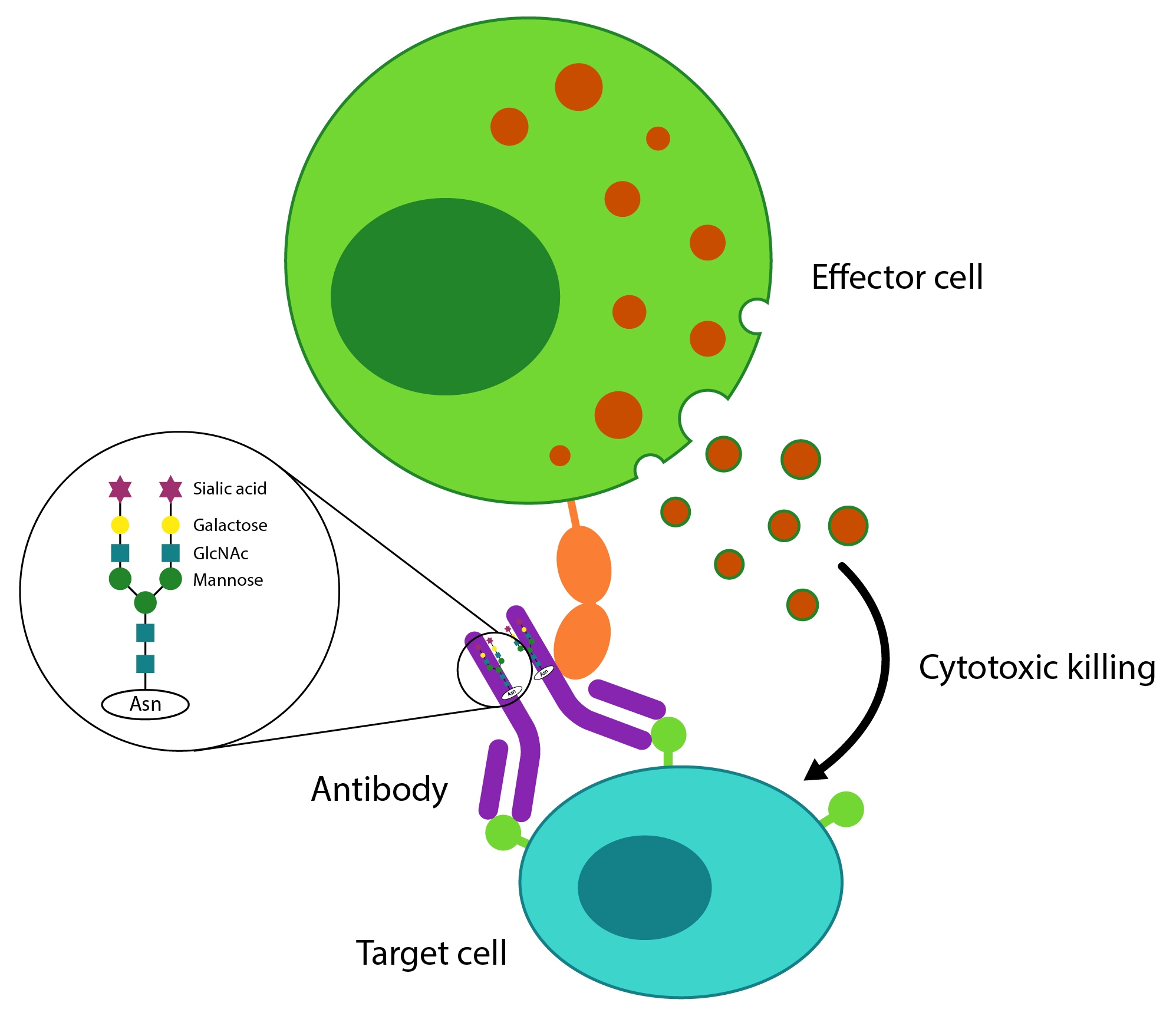
Figure 2: Antibody-dependent Cell Cytotoxicity immune response mediated by FcγRIIIa expressing effector cells: The antibody binds to a target cell via the antibody Fab region and to the FcγRIIIa receptor via the Fc region. This FcRIIIa-antibody Fc binding is enhanced by the absence of fucose on the Fc glycan, thus increasing efficacy.
Benefits of CHOSOURCE ADCC+ cell line
Well-characterized with extensive validation study conducted
The CHOSOURCE ADCC+ cell line has undergone extensive validation studies to confirm the genotype as well as functional performance. This ensures that the cell line can produce completely afucosylated proteins with predictable and reduced batch-to-batch variation with regards to product fucosylation.
Ease of use
- Processes for CHOSOURCE ADCC+ are easily transferable from existing CHOSOURCE GS KO processes.
- Both CHOSOURCE GS KO and ADCC+ cell banks have been manufactured following cGMP.
Comprehensive solution
CHOSOURCE ADCC+ cell line can be licensed in combination with CHOSOURCE TnT transposon technology. The partnership of CHOSOURCE ADCC+ cell line and CHOSOURCE TnT transposon technology may accelerate development timelines and reduce efforts, thanks to high reproducibility across pools and clones and high clonal stability.
Dedicated support team
With the CHOSOURCE ADCC+ cell line comes a dedicated commercial and technical team to provide support throughout the use of the platform. Comprehensive protocols, Cell History Pack and risk statement are available for our licensees and CHOSOURCE ADCC+ cell line is offered under a non-royalty-bearing commercial license with flexible terms and no media or third-party constraints.
The CHOSOURCE™ Platform is available for research, clinical, diagnostic, and commercialization applications under specific licenses from Revvity. The platform is also available for services under a service license from Revvity.