Why is cell viability measurement important?
In the recent decade, cellular therapy has become one of the main methods for treating various cancer diseases. The ultimate goal is to retrieve the immune cells (i.e. T cells, NK cells) and genetically modify them to recognize and eliminate the cancer cells in patients. Therefore, it is critical to accurately measure cell viability, otherwise it may lead to inefficacy or induce unwanted autoimmune responses in patients undergoing therapeutic treatments (1-3).

The U.S. Congress has also recognized the importance of cell counting and cell viability measurement standards in the 21st Century Cures Act for cell and gene therapy. On March 31, 2014, the U.S. Food and Drug Administration (FDA) hosted a workshop entitled “Synergizing Efforts in Standards Development for Cellular Therapies and Regenerative Medicine Products”. With the inclusion of the National Institute of Standards and Technology (NIST) and other stakeholders, cell counting and viability measurement assurance were identified as important standards for improving the quality of cell therapy products (4).
What is Trypan blue?
Trypan blue is an azo dye (m.w. 960 Da) that has been used for cell viability measurements for over a century (5,6). It can concentrate in membrane-compromised dead or dying cells, but is excluded from membrane-impermeable live cells (7,8). Although many issues have been documented for Trypan blue, such as protein aggregation (9-11), limited counting time window (12), and inaccurate measurement when viability is less than 80% (13-15), it remains the go-to viability dye.
Since 2012, we have published three articles detailing cell counting issues with Trypan blue staining. The first publication demonstrated that brightfield counting with Trypan blue can over-count live peripheral blood mononuclear cells (16). The second publication quantitatively showed that Trypan blue can transform dead cells to dim and diffuse shapes for Jurkat cells and primary mouse splenocytes, which caused over-estimation of viability when cell viability falls below 80% (15). We have further investigated the formation of the diffuse objects in the third article that was recently published (17).
What are these dim and diffuse objects formed after Trypan blue staining?
Traditionally, these diffuse objects are difficult to see in microscopy images, causing researchers to under-count dead cells and over-estimate viability. In contrast, the optical components in the Cellometer™ instruments are able to clearly image the dim and diffuse objects in Jurkat, mouse splenocytes, and human PBMCs.
In this work, we recorded the interaction between Trypan blue and cells in real-time to prove the rupturing of cells changing to the diffuse objects. With the use of fluorescent viability dye (propidium iodide, PI) that stained the nuclei of membrane-compromised dead cells, we demonstrated that these diffuse objects contained a nucleus.
Finally, we hypothesized that increase in osmotic pressure led to the rupturing of the dead or dying immune cells. This hypothesis was tested by changing the buffer concentrations and quantifying the size and color change of the Trypan blue-stained cells.
The visual and quantitation evidence recorded in these experiments revealed that Trypan blue can induce the formation of these diffuse objects and ultimately leads to over-estimation of viability, which may have significant effects for downstream cellular therapy assays.
Initial visual observation of the dim and diffuse objects in Trypan blue-stained Jurkat cells
We showed that a 2-day-old Jurkat cell sample (stored in the refrigerator for 2 days) stained with Trypan blue displayed 3 distinct morphological populations (Figure 1) in the brightfield images captured by Cellometer AutoT4.
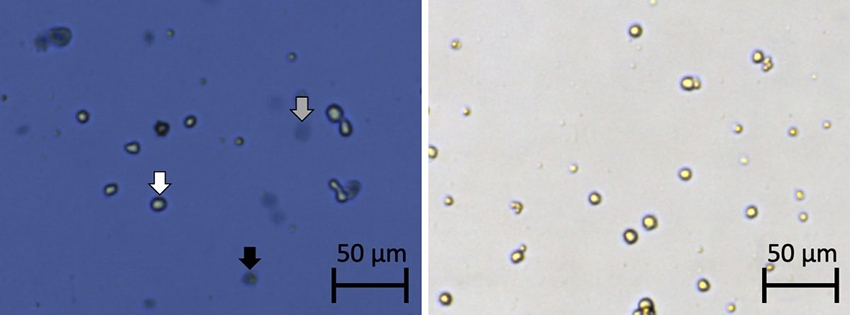
Figure 1. Brightfield cell images taken using the Cellometer Auto T4. Cells stained with Trypan blue (left) and cells stained with PI (right).
- Cells that were bright, round, and plump were live cells not stained by Trypan blue.
- Cells that were blue, dark, and compact were dead and stained by Trypan blue.
- Large, dim, and diffuse objects were potentially dead or dying cells that were affected by Trypan blue.
In addition, we have observed an increase in the diffuse objects after staining Jurkat cells, mouse splenocytes, and human PBMCs with Trypan blue when cells were collected at different time points during refrigerator storage (Figure 2). Interestingly, Jurkat cells stained with PI did not exhibit the same morphological changes.
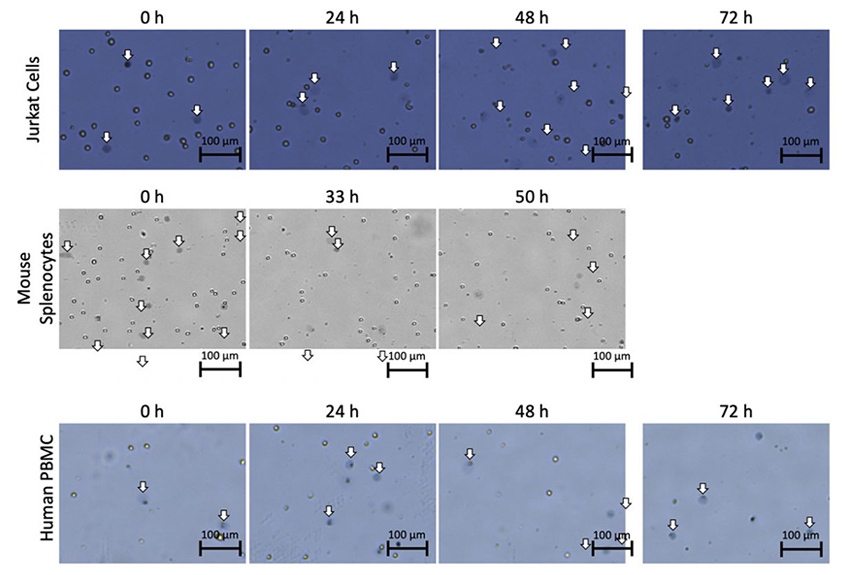
Figure 2. Stained Jurkat cells, mouse splenocytes, and human PBMCs.
Trypan blue rupturing dead or dying Jurkat cells
To video capture the staining process of Trypan blue, we set up a stage with the CHT4 cell counting chamber to allow slow diffusion of Trypan blue into the chamber (Figure 3). First, the chamber was filled with 20 µL of 2-day old Jurkat cells. Next, one of the air release ports was taped over with scotch tape to prevent movement of liquid. Finally, a droplet of 10 µL of Trypan blue was pipetted to the other air release port. The Cellometer AutoT4 was then used to capture images and videos at the specific location on the cell counting chamber.
In the first captured video, we observed that as Trypan blue diffused across the screen from bottom to top, it changed the dead or dying cells to large diffuse objects within 45 seconds after interacting with the Trypan blue. The next video showed Trypan blue diffusing from top to bottom and showed cell rupturing within ~2 seconds and disappearing toward the end. In the video, some dead cells were rendered dark and compact, while others changed to dim and diffuse.
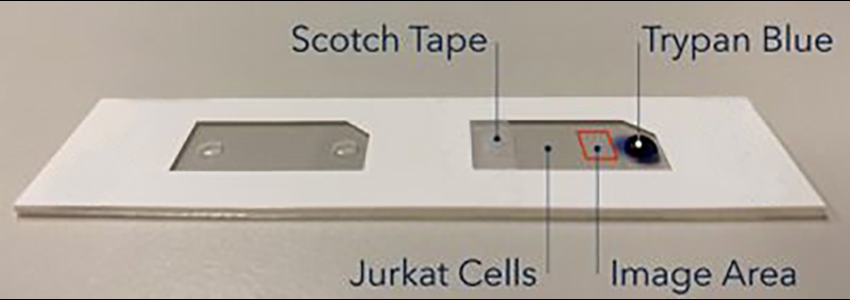
Figure 3. CHT4 cell counting chamber setup.
The Trypan blue stained dim and diffuse objects are in fact cells
To prove that the dim and diffuse objects were actually dead cells, we used PI to stain the nuclei of the cells. If PI fluorescence did not exist, it indicated there was no DNA materials in the object. We stained the 2-day old Jurkat cells with both Trypan blue and PI, which showed an overlap between the PI fluorescent signal and Trypan blue positive objects, indicating that the diffuse Trypan blue stained objects were in fact dead cells (Figure 4a). Merged brightfield and red fluorescence images show that PI-positive cells retained a compact appearance and did not appear to exhibit the diffuse morphology of Trypan blue positive cells (Figure 4b), which suggests that PI does not have the same morphological effects as Trypan blue.
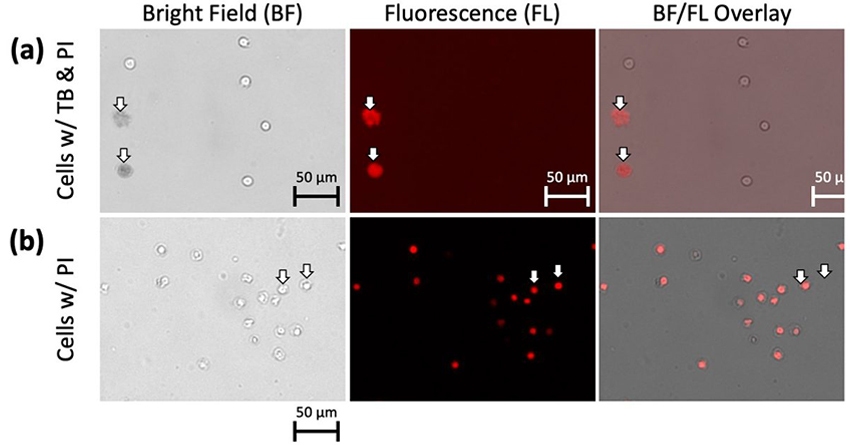
Figure 4. 2-day old Jurkat cells with both Trypan blue and PI.
Trypan blue-based cell rupturing is caused by high osmotic pressure and water influx
We believe that cell rupturing may be due to a higher osmotic pressure causing rapid influx of water. Different PBS concentrations had obvious effects on Trypan blue staining (Fig 5a). The 2-day old Jurkat cells were resuspended in different concentrations of PBS from 0.25X to 10X. The 0.25X PBS led to increases in the number and size of ruptured cells. The cells incubated in 1X to 6X PBS showed similar diffuse morphology, but with increasing darkness. The 8X and 10X PBS formed precipitation, presumably due to high salt concentration (18). The resulting images did show reduced number and size of ruptured cells, with a significant increase in darkness. The PBS concentration-dependent light intensity measurement showed a reduction in light intensity as the PBS concentration increased (Fig 5b), which strongly indicated that cells ruptured due to water influx during Trypan blue staining.
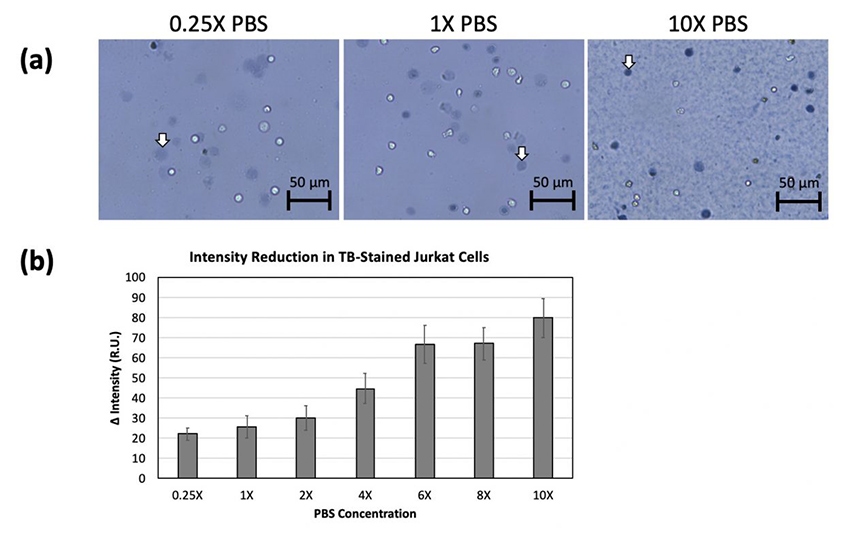
Figure 5. 2-day old Jurkat cells were resuspended in different concentrations of PBS.
The staining of cells with Trypan blue may rapidly increase the number of negatively charged residues on cytoplasmic proteins (8,19), which attracted more positively charged ions such as sodium, leading to high water influx and rupture of the already fragile cell membrane and cytoskeleton (3,20-22).
Heat-killed dead cells are not ruptured by Trypan blue
Interestingly, the heat-killed Jurkat cells stained with Trypan blue did not exhibit diffused morphology (15,23). In the video, the heat-killed cells did not rupture by Trypan blue staining, which may be caused by the denaturing of membrane proteins during boiling, resulting in cell membrane hardening that can withstand the influx of Trypan blue and water (24,25). Trypan blue appeared to enter the dead cells and increase the darkness without rupturing the membranes, where cell size only increased by less than 1 µm. The final still images showed no expansion of Trypan blue stained cells (Figure 6).
Concluding remarks
Since the approval of two chimeric antigen receptor (CAR) T cell therapies by the FDA (26) in 2017, cellular therapies have become important cancer treatments due to their high efficacy. Accurate cell counting and viability (FDA recommendation of greater than 70% viability for cellular therapy products (27)) is required to ensure proper dosages are administered and minimize the risk of an autoimmune response (1,2). We hypothesize that transferring a large amount of dead cells to the patients may pose potential side effects such as cytokine release syndrome (28).
It is important for biologists to select the most accurate cell counting method that is fit-for-purpose, especially for cellular therapy products such as CAR-T, where cell number and viability significantly affect treatment efficacy. Cells that rupture during Trypan blue staining are difficult to see under brightfield imaging and can be undercounted, leading to an overestimation of cell viability. We suggest using fluorescent nuclear staining methods that may estimate cell viability more accurately (15,16,23,29-31).
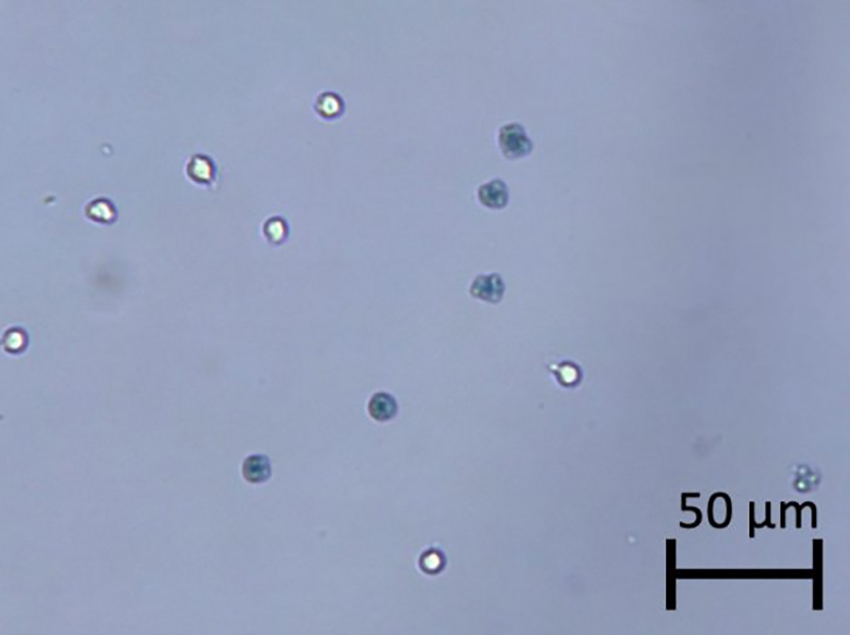
Figure 6. Heat-killed dead cells stained with Trypan blue
For research use only. Not for use in diagnostic procedures.
References:
- Nagata S, Hanayama R, Kawane K. Autoimmunity and the Clearance of Dead Cells. Cell 2010;140(5):619-630.
- Rock KL, Kono H. The inflammatory response to cell death. Annual Review of Pathology 2008;3:99-126.
- Zhang Y, Chen X, Gueydan C, Han J. Plasma membrane changes during programmed cell deaths. Cell Research 2018;28:9-21.
- Arcidiacono JA, Bauer SR, Kaplan DS, Allocca CM, Sarkar S, Lin-Gibson S. FDA and NIST collaboration on standards development activities supporting innovation and translation of regenerative medicine products. Cytotherapy 2018;20:779-784.
- Piccinini F, Tesei A, Arienti C, Bevilacqua A. Cell Counting and Viability Assessment of 2D and 3D Cell Cultures: Expected Reliability of the Trypan Blue assay. Biological Procedures Online 2017;19(8):1-12.
- Tran S-L, Puhar A, Ngo-Camus M, Ramarao N. Trypan Blue Dye Enters Viable Cells Incubated with the Pore-Forming Toxin HlyII of Bacillus cereus. PLOS One 2011;6(9):e22876.
- Chung DM, Kim JH, Kim JK. Evaluation of MTT and Trypan Blue assays for radiation-induced cell viability test in HepG2 cells. International Journal of Radiation Research 2015;13(4):1-6.
- Wainwright M. Dyes, trypanosomiasis and DNA: a historical and critical review. Biotechnic and Histochemistry 2010;85(6):341-354.
- Beaudoin AR, Kahkonen D. The Effect of Trypan Blue on the Serum Proteins of the Fetal Rat. The Anatomical Record 1963;147(3):387-395.
- Black L, Berenbaum MC. Factors Affecting the Dye Exclusion Test for Cell Viability. Experimental Cell Research 1964;35:9-13.
- Gao H-W, Zhao J-F. Interaction of Trypan Blue with Protein and Application. Journal of the Chinese Chemical Society 2003;50:329-334.
- Kwok AKH, Yeung C-K, Lai TYY, Chan K-P, Pang CP. Effects of trypan blue on cell viability and gene expression in human retinal pigment epithelial cells. British Journal of Ophthalmology 2004;88(12):1590-1594.
- Mascotti K, McCullough J, Burger SR. HPC viability measurement: trypan blue versus acridine orange and propidium iodide. Transfusion 2000;40(6):693-696.
- Tennant JR. Evaluation of the Trypan Blue Technique for Determination of Cell Viability. Transplantation 1964;2(6):686-694.
- Chan LL-Y, Kuksin D, Laverty DJ, Saldi S, Qiu J. Morphological observation and analysis using automated image cytometry for the comparison of trypan blue and fluorescence-based viability detection method. Cytotech. 2014;DOI 10.1007/s10616-014-9704-5.
- Chan LLY, Laverty DJ, Smith T, Nejad P, Hei H, Gandhi R, Kuksin D, Qiu J. Accurate measurement of peripheral blood mononuclear cell concentration using image cytometry to eliminate RBC-induced counting error. Journal of Immunological Methods 2013;388(1-2):25-32.
- Chan LL-Y, Rice WL, Qiu J. Observation and quantification of the morphological effect of trypan blue rupturing dead or dying cells. PLoS One 2020;15(1):e0227950.
- Sarma KD, Ray D, Antony A. Improved sensitivity of trypan blue dye exclusion assay with Ni2+ or Co2+ salts. Cytotechnology 2000;32:93-
- Cannon J, Kim D, Maruyama S, Shiomi J. Influence of Ion Size and Charge on Osmosis. Journal of Physical Chemistry B 2012;116(14):4206-4211.
- Bursch W, Hochegger K, Török L, Marian B, Ellinger A, Hermann RS. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. Journal of Cell Science 2000;113:1189-1198.
- Costa Ed, Rodrigues EB, Dib E, Penha FM, Furlani BA, O. Magalhães J, Maia M, Meyer CH, Miranda Ad, Farah ME. Vital Dyes and Light Sources for Chromovitrectomy: Comparative Assessment of Osmolarity, pH, and Spectrophotometry. Investigate Ophthalmology and Visual Science 2009;50(1):385-391.
- Lodish H, Berk A, Zipursky SL. Section 15.8 Osmosis, Water Channels, and the Regulation of Cell Volume. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000.
- Chan LL, Wilkinson AR, Paradis BD, Lai N. Rapid Image-based Cytometry for Comparison of Fluorescent Viability Staining Methods. Journal of Fluorescence 2012;22(5):1301-1311.
- Bischof JC, Padanilam J, Holmes WH, Ezzell RM, Lee RC, Tompkins RG, Yarmush ML, Toner M. Dynamics of Cell Membrane Permeability Changes at Supraphysiological Temperatures. Biophysical Journal 1995;68:2608-2614.
- Mackey BM, Miles CA, Parsons SE, Seymour DA. Thermal denaturation of whole cells and cell components of Escherichia coli examined by differential scanning calorimetry Journal of General Microbiology 1991;137:2361-2374.
- Yip A, Webster RM. The market for chimeric antigen receptor T cell therapies. Nature Reviews Drug Discovery 2018;17:161-162.
- U.S.-Department-of-Health-and-Human-Services, Food-and-Drug-Administration, Center-for-Biologics-Evaluation-and-Research. Guidance for FDA Reviewers and Sponsors – Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs) 2008.
- Hasegawa K, Hosen N. Chimeric antigen receptor T cell therapy for multiple myeloma. Inflammation and Regeneration 2019;39(10):1-5.
- Altman SA, Randers L, Rao G. Comparison of Trypan Blue Dye Exclusion and Fluorometric Assays for Mammalian Cell Viability Determinations. Biotechnology Progress 1993;9:671-674.
- Solomon M, Wofford J, Johnson C, Regan D, Creer MH. Factors influencing cord blood viability assessment before cryopreservation. Transfusion 2010;50(4):820-830.
- Chan LL-Y, McCulley KJ, Kessel SL. Assessment of Cell Viability with Single-, Dual-, and Multi-Staining Methods Using Image Cytometry. Methods in Molecular Biology. Volume 1601; 2017. p 27-41.

































