The clinical success rate of new oncology drugs is only 3.4% compared to 20.9% in other disease types (Wong et al.,2019). One contributing factor to this issue is the testing systems used, with two-dimensional (2D) monolayer assay formats as the traditional mainstay of high throughput screening. Although 2D monolayer assays have identified many successful drugs, it is increasingly recognized that they do not accurately model critical aspects of the three-dimensional (3D) tumor environment. (Breslin et al., 2013). Therefore, the adoption of high throughput screening approaches using 3D assays to complement 2D approaches could substantially improve the prediction of clinical outcomes and reduce the high failure rate of cancer drugs in clinical trials.
What are the advantages of 3D cell models in high throughput screening?
Using 3D cell models for high throughput screening (HTS) offers several potential advantages:
- Enhanced physiological relevance: 3D cell models such as spheroids and organoids better mimic the architecture and microenvironment of human tissues, including critical cell-cell and cell-matrix interactions. This closer approximation to actual human tissue conditions can lead to more accurate drug efficacy and toxicity assessments.
- Improved predictive accuracy: 3D cell models' enhanced physiological relevance can provide more predictive data for in vivo responses. This enhances the likelihood of identifying effective and safe drug candidates early in development, reducing costly late-stage failures.
- Simulation of complex biological processes: These models are better suited to studying complex biological processes such as tumor growth, metastasis, and organ-specific functions, providing deeper insights into disease mechanisms and treatment effects.
- Reduction in animal testing: By providing more accurate in vitro models, 3D systems can reduce the reliance on animal testing, addressing both ethical concerns and high costs which can be associated with animal studies.
- Realistic drug penetration and distribution: In 3D cell models, drug diffusion and distribution can more closely mimic in vivo conditions, critical for understanding pharmacokinetics and pharmacodynamics, particularly in solid tumors.
- Suitability for high throughput screening: Advances in automation and imaging technologies have enabled 3D cell models in high throughput formats, facilitating large-scale screening efforts.
- Investigation of drug resistance mechanisms: 3D cell models allow for a more accurate study of drug resistance mechanisms, such as the effects of the extracellular matrix on cellular signaling pathways.
- Longer culture duration: 3D cultures often allow longer-term studies, which is essential for assessing chronic toxicity and long-term drug effects.
What's involved with a spheroid-based 3D assay?
Many 3D assays have been described, but by their very nature, most are typically complex, involving multiple cell types, extracellular scaffolds, and complex image-based readouts: they can be challenging to work with at scale.
By contrast, 3D tumor spheroids produced by plating single cell suspensions of tumor cell lines in low anchorage U-bottom microplates, such as Revvity’s CellCarrier™ Spheroid ULA microplate, in liquid growth media are amenable to straightforward add-and-read plate reader-based viability assays such as Revvity's ATPlite™ 3D. This relative simplicity makes spheroid assays the most amenable to standardization for routine high-throughput screening.
What can spheroids offer?
Increasingly, data indicate that tumor spheroid models can accurately mimic key aspects of the tumor microenvironment, including 3D cell-to-cell interactions and nutrient and oxygen gradients (Breslin et al., 2013). They also uncover unique insights into drug responses that probably wouldn't be discovered using traditional 2D assays.
For example, Jeff Settleman and colleagues at Genetech elegantly applied 3D spheroid and standard 2D assays to identify a potential drug combination strategy to treat pancreatic cancer. 'We screened a large panel of anticancer agents using a two-dimensional monolayer culture system and a three-dimensional spheroid culture system. These results reveal a combination drug treatment that might be effective in pancreatic cancer' (Sahu et al., 2017).
These and our in-house data support the hypothesis that 3D drug panel screening could help predict which patient populations most respond to a drug or biologic. As a result, Revvity has established a panel of approximately 200 cell lines to screen single agents and drug combinations for use in high-throughput assays (Scales et al.,2018).
The combination of 2D screening approaches with 3D assays improves the modeling of the tri-dimensional tumor microenvironment's mechanical, biochemical, and cell-to-cell signaling for enhanced clinical response predictions. The 2D and 3D OncoSignature™ screening offerings are run continuously with monthly data deliveries to allow for flexible onboarding and compound submission. Screens can be run across a diverse, clinically relevant 200 (3D) or 300 (2D) cell line panel or a more focused, tissue-specific panel such as lung, lymphoma, head and neck, or breast.

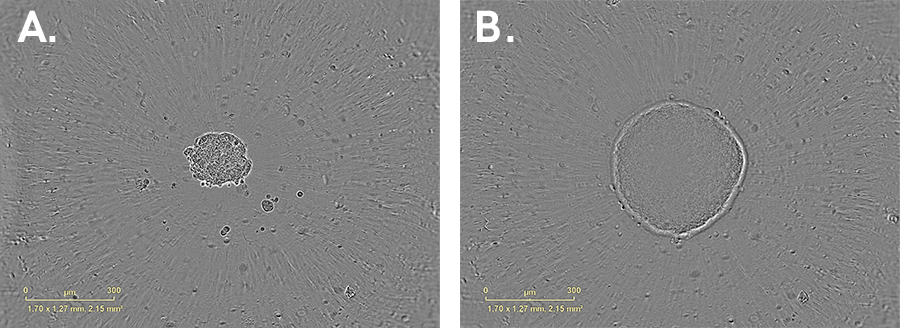
Growth of a HT-29 (colorectal cell line) spheroid cultured in 384-well U- bottom ULA plate. Images of the same spheroid at (A) 1 day and (B) 8 days post-seeding. Cells were seeded at a density of 750 cpw in Mc Coy’s 5A media with 10% FBS. Brightfield images. 10x magnification
Find out more about our 3D cell panel screening services to complement 2D assay results using the OncoSignature™ platform.
References:
- Breslin, S., & O’Driscoll, L. (2013). Three-dimensional cell culture: the missing link in drug discovery. Drug discovery today, 18(5-6), 240-249.
- Sahu, N et al (2017) Cotargeting of MEK and PDGFR/STAT3 pathways to treat pancreatic ductal adenocarcinoma. Molecular Cancer Therapeutics, Vol 16(9), pp1729-1738.
- Scales, T et al (2018) High-Throughput cell panel and organoid screening in 3D. Poster presentation, ELRIG London, October 2018.
- Wong, C et al (2019) Estimating clinical trial success rates and related parameters. Biostatistics Vol 20 (2), pp273-286.
For research use only. Not for use in diagnostic procedures.