Inflammation of the digestive tract wall due to an overactivation of the digestive immune system is a characteristic of inflammatory bowel diseases. The exact etiology of IBD is still unknown but several risk factors are suspected, including environmental and genetic factors [1] (Figure 1). Studies of the genome of IBD patients have enabled the identification of more than 150 genes predisposing to these diseases. These genes are involved in different pathophysiological mechanisms such as the recognition of microbes, lymphocyte activation, signaling and production of cytokines, and defense of the intestinal epithelium [2,3,4].

Figure 1: The etiological puzzle of IBD
Inflammatory Bowel Diseases and some figures
An IBD can occur at any age, but a high prevalence has been observed between 14 and 24 years old [7] and both sexes are affected. In 2017, there were 6.8 million cases of IBD globally. The average prevalence rate is 84.3 per 100 000 of population [5], but this frequency varies significantly depending on the country. A higher frequency is found in industrialized regions like North-West Europe or the USA. At the national level, the USA had the highest age-standardized prevalence rate with 465 per 100 000 of population, followed by the UK with 450 per 100 000 [5]. Moreover, the risk of being diagnosed as having an IBD is multiplied by 4 to 20 if your parents have already suffered from an IBD.
IBDs include Crohn’s Disease (CD) and Ulcerative disease (UC). For both, there are no curative treatments at present. The main goals of treatment are to reduce symptoms, known as inducing remission (a period without symptoms), and then to maintain remission [6]. There are many researchers investigating this topic, and they have developed different strategies to move IBD knowledge forward in order to improve the daily life of those affected. Revvity teams are working on kits to help researchers in implementing their different strategies.
In a strategy to increase knowledge of IBD pathogenesis
In 2019, Irina V. Tcymbarevich et al [10]. published an article which showed relevant results on the impact of rs8005161 polymorphism on the G protein-coupled receptor. The gene encoding G-protein-coupled receptor 65 (GPR65) has recently been reported to be a genetic risk factor for IBD. It was found that an extracellular acidification activates GPR65, which is in charge of cAMP and Rho signaling pathway stimulation.
To check their hypotheses, the researchers decided to work on CD14+ cells isolated from blood samples. The samples used were either from IBD patients (carrying rs8005161 TT or CT or CC) and non-IBD patients (carrying rs8005161 CC/WT). The authors used the HTRF cAMP dynamic kit to monitor the accumulation of cAMP induced by GPR65 activation at different pH levels (Figure 2)
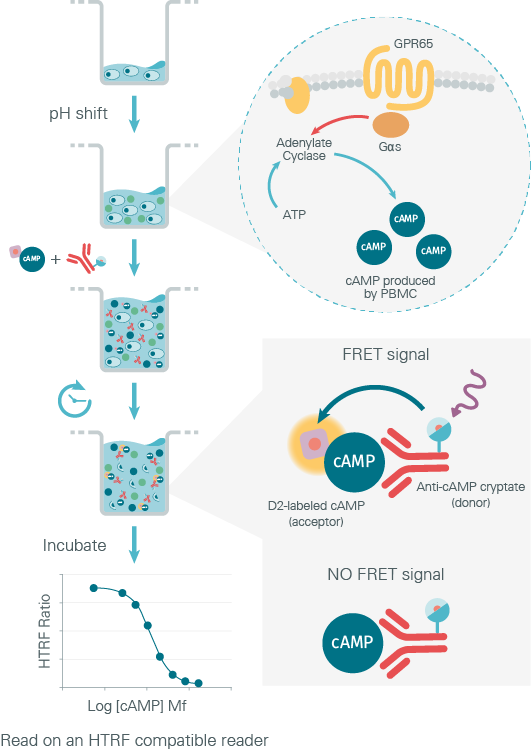
Figure 2: Principle of GPR65 functional assay using the HTRF cAMP kit
The results of the experiment showed that the cAMP level increases in PBMCs from all groups (healthy vs IBD samples) upon an extracellular acidification. So, Irina V. Tcymbarevich and her team concluded that no significant differences between IBD TT, CT, CC and non IBD WT/CC were detected in acidic pH induced cAMP production. Under the conditions tested, the genotype variant of GPR65 had no influence on the GPR65/Gs/cAMP pathway.
In a drug discovery strategy for IBD treatment
Retinoic Acid Related Orphan Nuclear Receptor gamma T (RORγT) is new in the list of contributors to the pathogenesis of IBDs. It is a specific transcription factor for IL-17 expressing cells. Leo R. Fitzpatrick et al [8]. are working on VPR-254, a selective in vitro inhibitor of RORγT. An article published in 2019 described a study to determine if ex vivo treatment with VPR-254 reduced relevant cytokine (especially IL-17) secretion from the colonic strips of mice with colitis.
To evaluate if VPR-254 specifically inhibits RORγT driven IL-17 secretion, the authors used HTRF cytokine kits to quantify IL-17. They concluded that VPR-254 reduced the production of key pro-inflammatory cytokines IL-17 in ex-vivo.
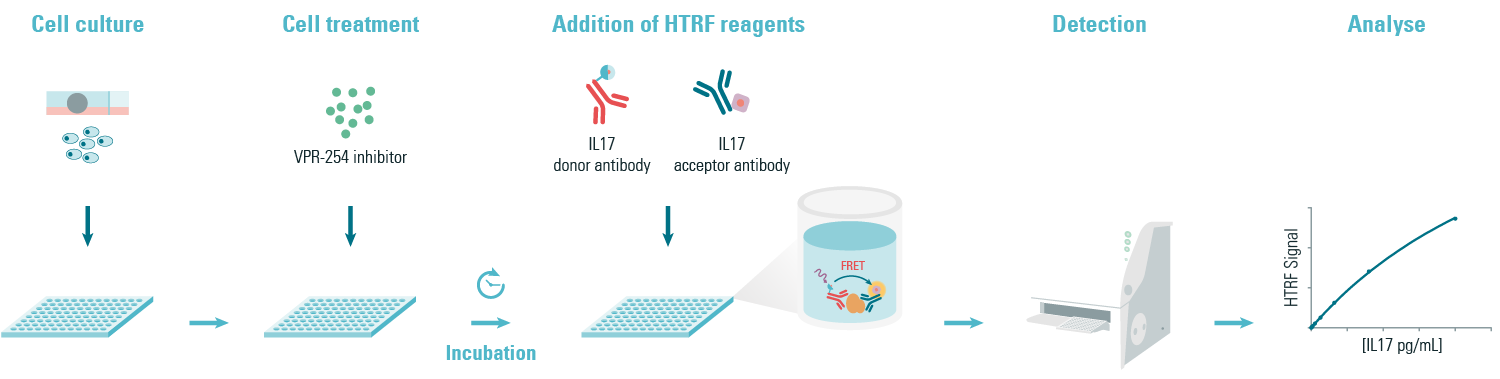
Figure 3: Principle of the HTRF IL17 sandwich assay
In a strategy to improve IBD management
UC is characterized by episodes of relapse and remission. Distinguishing a refractory UC patient in a remission state from a patient who is not in a refractory period remains difficult.
Shinsaku Hamanaka et al. [9] decided to investigate the use of novel biomarkers to do this. The discovery of novel clinical indicators could contribute to preventing the overuse of corticosteroids for treatment.
To analyze the serum antibody titers with candidate protein antigens, the researchers used AlphaLISA™ technology (Figure 5). The experiments were carried out on healthy people, and on patients with UC and patients with CD. Based on 66 candidates, they concluded that the serum titers of anti-PARG and anti-PRR13 antibodies were more elevated in patients with refractory UC than in patients with non-refractory UC. According to these experiments, anti-PARG and anti-PRR13 antibodies appear to be biomarkers for refractory UC and could play a key role in IBD treatment strategy.
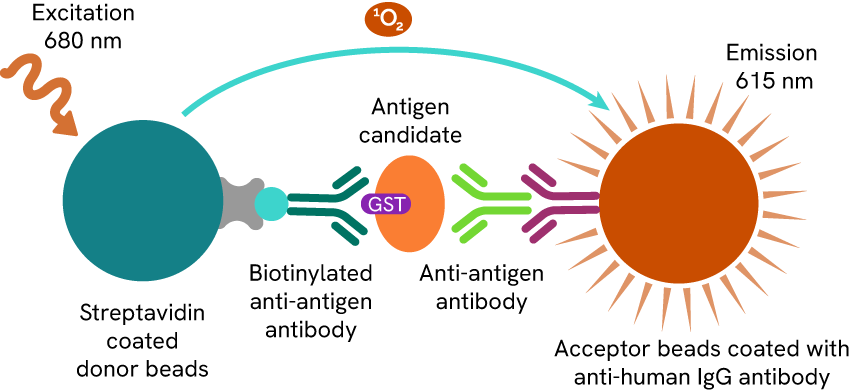
Figure 4: AlphaLISA quantification method
These three examples (and others not cited) can help you to imagine how user-friendly solutions like AlphaLISA and HTRF™ can be applied in the context of IBD research.
If you would like to read the full story, and explore the data further, you can download the review Improve Drug Discovery on Inflammatory Bowel Disease with HTRF and ALPHA solutions.
For research use only. Not for use in diagnostic procedures.
References:
- Nitzan O, Elias M, Peretz A, et al. Role of antibiotics for treatment of inflammatory bowel disease. World J Gastroenterol 2016 ; 22 : 1078-87.
- Dutta AK, Chacko A. Influence of environmental factors on the onset and course of inflammatory bowel disease. World J Gastroenterol 2016; 22: 1088-100.
- Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol 2010; 6: 339-46.
- Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol 2015; 12: 205-17
- Mohsen Naghavi. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017 – Lancet Gastroenterol Hepatol 2020; 5: 17–30
- Ulcerative Colitis – Treatment – NHS
- Revue générale des maladies intestinales inflammatoires chroniques. (Tcymbarevich et al., 2019) (Hamanaka et al., 2018) (Fitzpatrick et al., 2020)
- Fitzpatrick, L. R., et al., (2020). VPR-254: An inhibitor of ROR-gamma T with potential utility for the treatment of inflammatory bowel disease. Inflammopharmacology, 28(2), 499–511.
- Hamanaka, S., et al., (2018). Investigation of novel biomarkers for predicting the clinical course in patients with ulcerative colitis. Journal of Gastroenterology and Hepatology, 33(12), 1975–1983.
- Tcymbarevich, I. V., et al., (2019). The impact of the rs8005161 polymorphism on G protein-coupled receptor GPR65 (TDAG8) pH-associated activation in intestinal inflammation. BMC Gastroenterology, 19(1), 2.