Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease affecting motor neurons (MN). The disease is characterized by moderate and progressive dysfunction and loss of motor neurons. In the most advanced stages, patients develop symptoms of dyspnea and dysphagia. Most patients die of respiratory failure .
Key figures about Amyotrophic Lateral Sclerosis
ALS is considered a rare disease because of its extremely low prevalence, with 2 to 3 cases per 100,000 people. The cause is unknown in 90-95% of cases (sporadic ALS), and only 5-10% of cases are associated with known genetic mutations (familial ALS). Data from clinical studies show that ALS is linked to multiple mutations, the most common of which are C9orf72, SOD1 (superoxide dismutase 1), FUS (Fused in Sarcoma), and TDP-43 (TAR DNA Binding Protein 43), as well as enhanced neuroinflammation. Even in the absence of known genetic alterations, there is immune dysregulation .
Immune dysregulation – results in increased central and peripheral inflammation
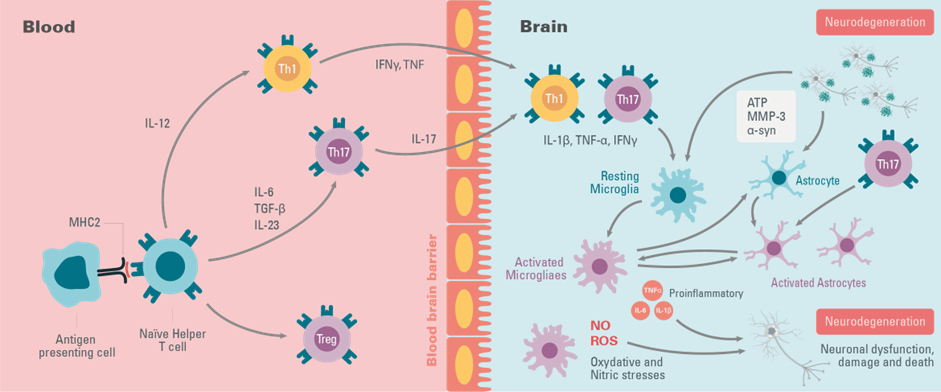
Although immune alterations do not trigger ALS, numerous studies indicate that activation of microglia in the central nervous system (CNS) occurs before or at the same time as the onset of clinical symptoms and increases throughout the disease. The in vivo activation state of cells involved in ALS is characterized by a continuum between the neuroprotective and neurotoxic states. As the disease progresses, astrocytes are activated, either through activated microglia or independently through the release of compounds from motor neurons. Thus, these glial cells express proinflammatory cytokines (TNF-α, IL-1β
 HTRF Human TNF-α Detection Kit, 96 Assay Points
Discover
, IL-12, IFN-γ, IL-6
HTRF Human TNF-α Detection Kit, 96 Assay Points
Discover
, IL-12, IFN-γ, IL-6
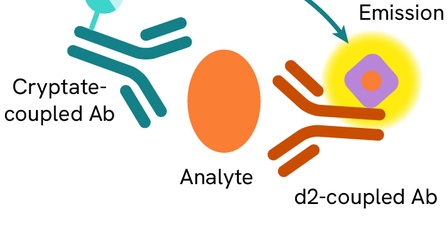 HTRF Human IL-6 Detection Kit, 96 Assay Points
Discover
), mitogenic factors (MCP-1, M-CSF), and neurotrophic factors (IGF-1), including molecules involved in oxidative stress (cyclo-oxygenase-2, ROS, NOS) that can promote a neurotoxic environment. When the critical threshold is reached, the pathological process becomes irreversible and leads to the death of non-autonomous motor neurons in ALS patients.
HTRF Human IL-6 Detection Kit, 96 Assay Points
Discover
), mitogenic factors (MCP-1, M-CSF), and neurotrophic factors (IGF-1), including molecules involved in oxidative stress (cyclo-oxygenase-2, ROS, NOS) that can promote a neurotoxic environment. When the critical threshold is reached, the pathological process becomes irreversible and leads to the death of non-autonomous motor neurons in ALS patients.
Neuroinflammation is not limited to the CNS, it needs a systemic inflammatory response to enable the local inflammation to take place. One study has shown elevated levels of TNF-α, IL-17
 HTRF Human IL-17A / IL17RA Binding Kit, 500 Assay Points
Discover
, IFN-γ factors, and peripheral immune cells, T cells, in the blood and CSF of patients. Neuroinflammation of the CNS therefore causes an increase in proinflammatory T-cell infiltration as the disease progresses. The hypothesis put forward is that due to motor nerve damage resulting from misfolded SOD1 and FUS, and TDP-43 aggregation
HTRF Human IL-17A / IL17RA Binding Kit, 500 Assay Points
Discover
, IFN-γ factors, and peripheral immune cells, T cells, in the blood and CSF of patients. Neuroinflammation of the CNS therefore causes an increase in proinflammatory T-cell infiltration as the disease progresses. The hypothesis put forward is that due to motor nerve damage resulting from misfolded SOD1 and FUS, and TDP-43 aggregation
 HTRF TDP-43 Aggregation Kit, 500 Assay Points
Discover
, specific antigens are released and processed by antigen-presenting cells (APCs). APCs are believed to induce the priming of specific T cells and their recruitment into the CNS, which promotes inflammation of the MNs, leading to their death .
HTRF TDP-43 Aggregation Kit, 500 Assay Points
Discover
, specific antigens are released and processed by antigen-presenting cells (APCs). APCs are believed to induce the priming of specific T cells and their recruitment into the CNS, which promotes inflammation of the MNs, leading to their death .

Figure 2: The key elements of immune system peripheral and central inflammation during ALS Disease
Mitochondrial dysfunction
Mitochondrial dysfunction associated with ALS correlates with stressors such as membrane depolarization, dysfunctions of the mitochondrial complex, mutagenic stress, and proteotoxicity. Mitochondrial damage causes an accumulation of PINK1 on the outer mitochondrial membranes and activates Parkin ligase by directly or indirectly phosphorylating Ubiquitin Ser65. This amplifies the signal for damage detection. Thus, the PINK1/Parkin complex induces fragmentation of the mitochondrial network by mitophagy as a physiological quality control response to mitochondrial injury. However, as the disease progresses, mitophagy fails to clear the damaged mitochondria; therefore, mitochondrial fragmentation is self-propagating. The mitochondria are sequestered by the autophagy machine, which fuses with a lysosome to degrade its contents.
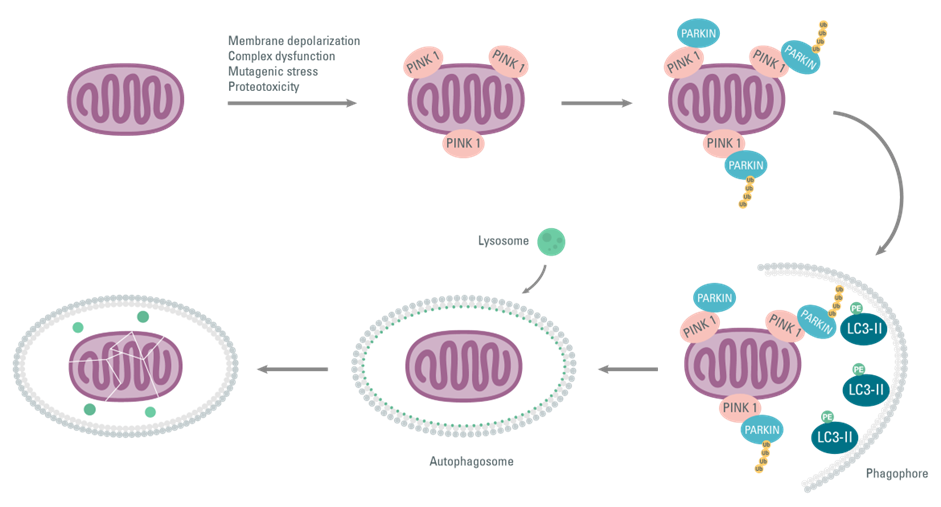
Figure 3: Activation of PINK1/PARKIN on mitochondrial membranes following various stresses, and causing mitophagy
Conclusion
These early or late inflammatory responses as well as mitophagy impairment have been similarly associated with other neurodegenerative diseases, such as Parkinson’s and Alzheimer’s Disease, and represent potential therapeutic targets.
Bibliography
- David R Beers, Stanley H Appel. Immune dysregulation in amyotrophic lateral sclerosis: mechanisms and emerging therapies. The lancet neurology. (18)30394-6.
- Elena Obrador, Rosario Salvador, Rafael López-Blanch. Oxidative Stress, Neuroinflammation and Mitochondria in the Pathophysiology of Amyotrophic Lateral Sclerosis. Antioxidants 2020,9,901.
- Santa Mammana, Paolo Fagone, Eugenio Cavalli. The Role of Macrophages in Neuroinflammatory and Neurodegenerative Pathways of Alzheimer’s Disease, Amyotrophic Lateral Sclerosis, and Multiple Sclerosis: Pathogenetic Cellular Effectors and Potential Therapeutic Targets. Int.J.Mol.Sci. 2018,19,831.
- Mengmeng Jin, Rene Günther, Katja Akgün. Peripheral proinflammatory Th1/ Th17 immune cell shift is linked to disease severity in amyotrophic lateral sclerosis. (2020) 10:5941.
- Emma F.Smith,Pamela J.Shaw, Kurt J.DeVos. The role of mitochondria in amyotrophic lateral sclerosis. The role of mitochondria in amyotrophic lateral sclerosis. Neurosciences letters 710 (2019) 132933.
For research use only. Not for use in diagnostic procedures.