Inflammation is a central part of the human organism’s self-defense arsenal, and greatly contributes to the efficiency of our immune system and responses. As a result, autoimmunity disorders are often found to stem from inflammatory pathway mis-regulation. This means these pathways have become a high-stake research topic as awareness of their roles in diseases such as rheumatoid arthritis, multiple sclerosis or inflammatory bowel disease has spread.
This article provides an insight into published studies about researchers’ investigations of inflammatory pathways with fluorescence assays, advancing our understanding and identifying potential new therapeutic compounds and strategies.
Immune cells and inflammation
T-cells and B-cells are star players of our immune responses, and are directly responsible for the elaboration of complex inflammatory signals and pathogen suppression. These behaviors are not innate, but depend on cascading signal transductions which monitor cell activation and behavior. In the past decades, the cells have grown more prominent in our understanding of autoimmunity, and managing their activating pathways is now a hot topic in our approach to such disorders.
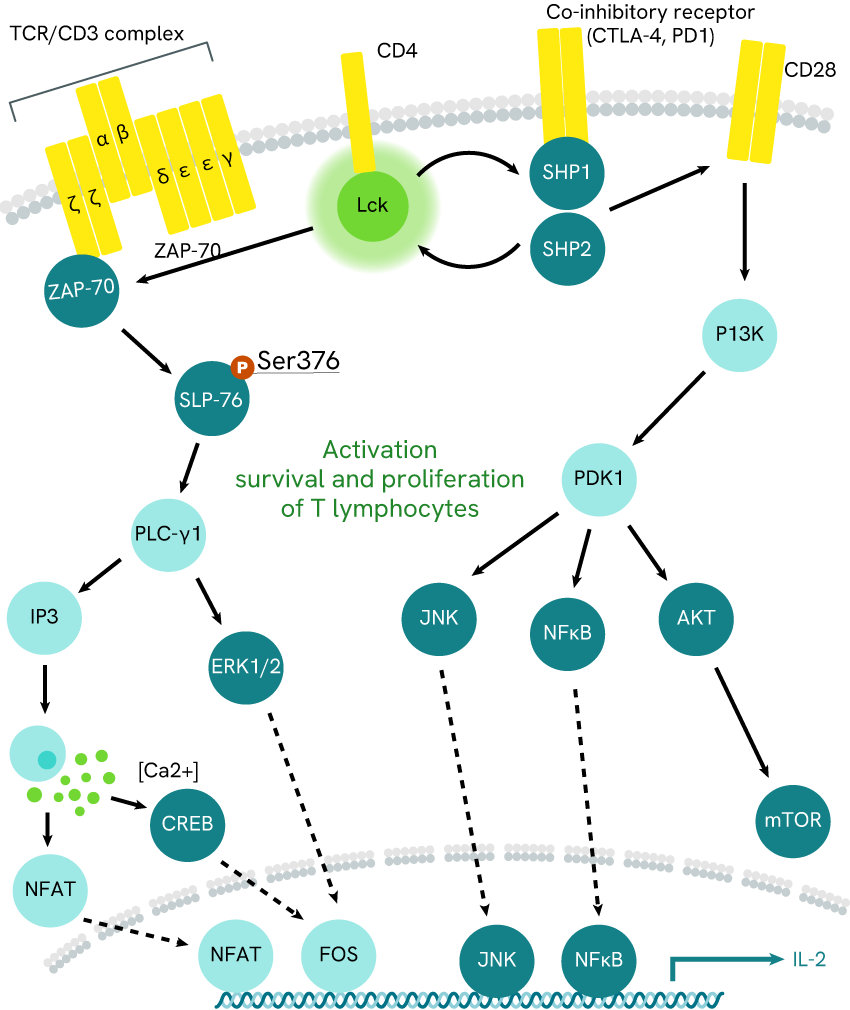
Examples include the work of McRae et al (2005) and Harbert et al (2008, who respectively addressed T-cell and B-cell activation pathways (fig. 1.a and 1.b, respectively).

Fig 1a: ZAP-7 pathway
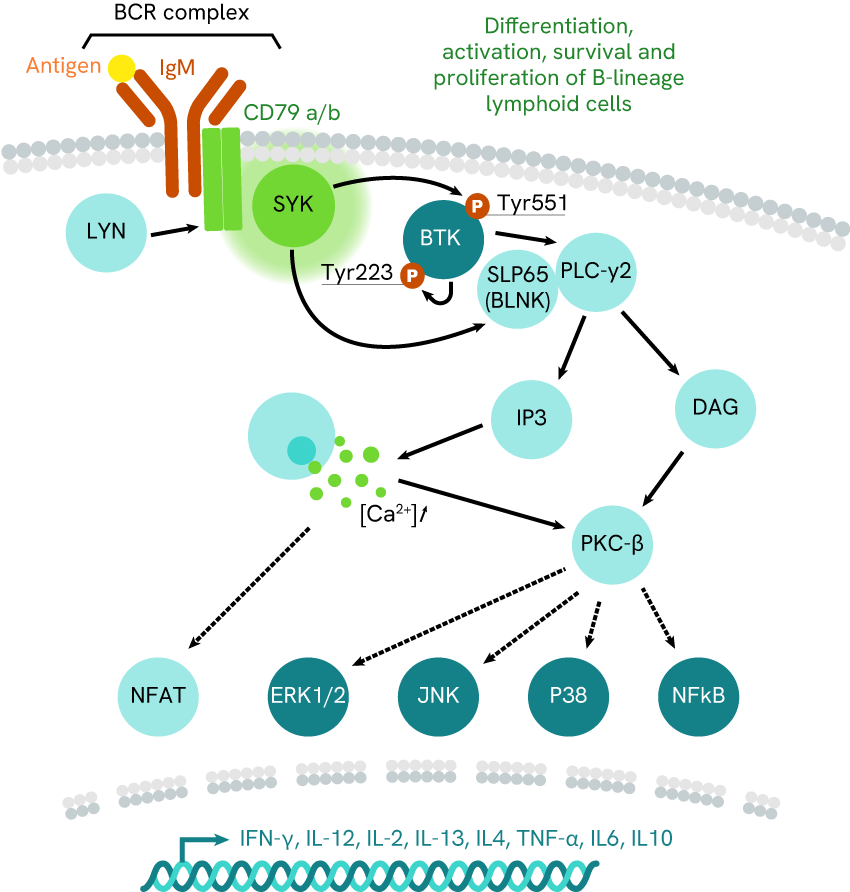
Fig 1b: BTK pathway
Zap-70 and BTK pathways, respectively monitoring CD4+ T-cell and B-cell activation. McRae’s team focused on Lck kinase inhibition, while Harbert’s team investigated SYK kinase activity.
The two studies relied on HTRF kinase assays to monitor the activity of Lck or SYK kinases.
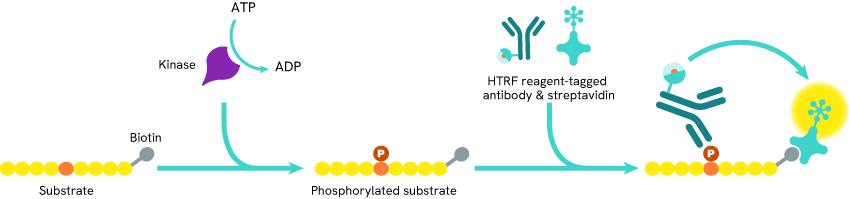
Fig. 2 HTRF kinase assay principle: Biotin-tagged substrate is incubated in presence of the kinase of interest and ATP, which leads to its phosphorylation. Some cryptate-coupled phosphorylation-specific antibodies and XL665-coupled streptavidin are added to the mix. The streptavidin binds to the biotin tag while the antibodies bind to the phosphorylation site, which brings the cryptate and XL665 into proximity and triggers FRET.
McRae’s team aimed to characterize the efficacy of their newly designed compound against a range of known inhibitors, and used the assay to determine the IC50 of each. Results were outstanding and showed the clear superiority of the new compound over all other inhibitors.
On the other hand, Harbert’s team sought to develop an HTS compatible assay for SYK inhibitor screening, and performed a set of experiments featuring the inhibitor Staurosporine in order to validate the accuracy of their assay. The Michealis-Menten analysis they performed on the results meant they could vouch for the relevance and consistency of their methods for further B-cell investigations.
The STING pathway and inflammasomes – an intricate and innate relationship
Immune cells are not the only cells contributing to inflammation, as most cells possess some innate strategies and assets empowering them to a greater or lesser extent.
Inflammasome are intracellular complexes enabling the detection of various cytosolic contaminants and triggering the massive release of pro-inflammatory IL-1 beta cytokine. They are also known to activate upon TLR stimulation. Elsewhere, cGAS-STING is a pathway whose role is to detect cytosolic contaminant DNA and trigger the release of pro-inflammatory IFN-beta.
A better understanding of the relationship between those two innate pro-inflammatory strategies could yield significant enhancements for therapeutic research surrounding auto-inflammatory and autoimmune disorders. In 2017, Gaidt et al investigated the ties between cGAS-STING and the AIM2 inflammasome in the context of cytosolic contaminant DNA detection. To do so, they used the HTRF IL-1 beta assay in experiments involving cGAS- or STING- cells, as well as the traditional inflammasome AIM2 inhibitor MCC950 and agonist Nigericin.
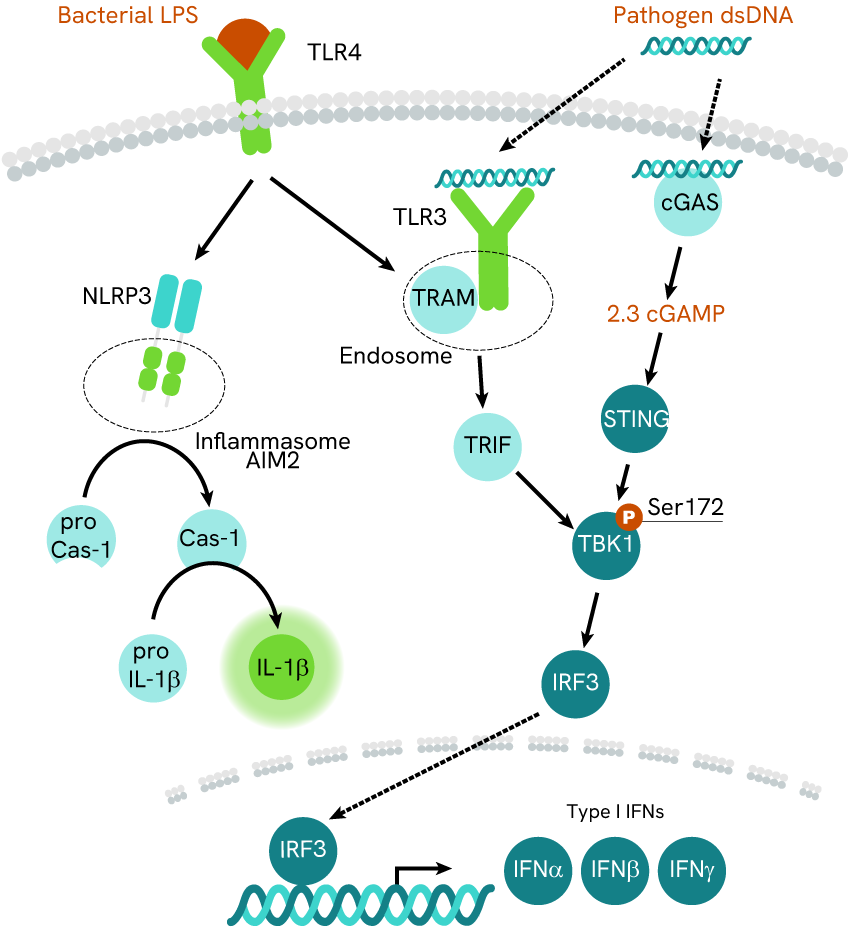
Fig.3 cGAS-STING axis and NLRP3 inflammasome pathways. Gaigt et al monitored IL-1beta as a marker of the inflammasome activity.
The results led the team to suggest the following: cGAS-STING is not needed for the activation of inflammasome AIM2, which can also be achieved through stimulation of its NLR3 part. However, DNA-mediated activation of the inflammasome appeared to rely heavily on cGAS-STING, suggesting a synergy of the two pathways to tackle contaminant DNA. Finally, even though cGAS-STING was necessary in such a context, it was not sufficient and the NLR3 part of the inflammasome remained as necessary for DNA-mediated activation as it was before.
For research use only. Not for use in diagnostic procedures.