The FDA’s approval of the first adeno-associated virus (AAV)-delivered gene therapy in 2017 marked a turning point in viral vector research. As of April 2024, four AAV-based therapeutics have received approval, with many more in the clinical pipeline.
AAVs have certain characteristics that make them effective as gene delivery vehicles. For instance, they require minimal genome size for replication which means that most of its genome can be replaced with the therapeutic DNA insert. AAVs are also versatile for targeting different tissues.
Yet, viral vector designs must have certain characteristics to be considered successful. For example, they need to be modifiable, safe, and stable. They also need to be quantifiable and scalable for large-scale production.
AAV vector manufacturing challenges
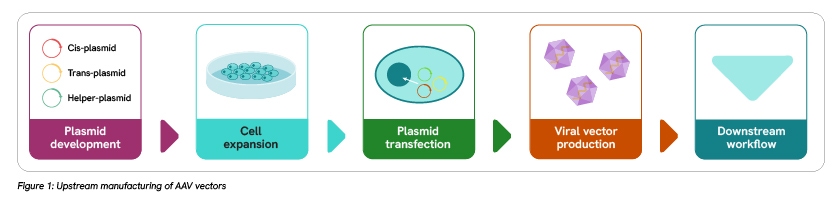
The scale up of AAV production workflows is one of the major bottlenecks in gene therapy research and clinical application. Two primary workflows are required for the production of AAV vectors: upstream vector production and downstream vector purification and enrichment.
The upstream workflow involves plasmid development, cell expansion, plasmid transfection, and vector production steps. Key challenges in the upstream workflow include improving yield and purity. For example, GMP-grade plasmid DNA must have a purity level of more than 95% and be free of process-related variants and impurities. Scaling up the cell expansion step has its own difficulties, including the risk of contamination during culture manipulation of adherent cells and inefficiencies of the AAV vector’s three-plasmid transfection system in suspension cell culture. These and other hurdles need to be addressed in the upstream workflow to enable more efficient vector manufacturing.

The downstream workflow also involves several steps, including cell lysis, removal of nucleic acids, solids, and host-cell proteins, separation of full gene-containing infectious viruses from empty, non-infectious viruses, and finally vector purification. Downstream processes are more standardized than upstream workflows, making them easier to scale up and more adaptable to different manufacturing platforms. But they are not without their challenges.
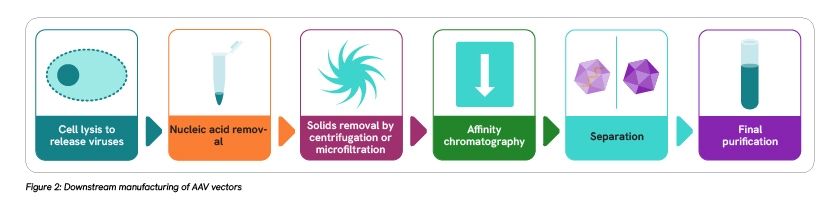
Considerations need to be made relating to methods for cell lysis, filtration, purification, and separation. For example, current cell lysis methods can place stress on the viral vector, which might impact the safety or efficacy of the final therapeutic product. Filtration is the most expensive process in the downstream workflow, so considerations need to be made around filter size to prevent vector loss. Effective separation methods are also required to reduce the presence of empty capsids, which can reduce the efficacy and safety of the final therapeutic product.
Additionally, developers need to ensure AAV vector stability during manufacturing and storage. Challenges include the potential degradation, denaturation, aggregation, or oxidation of the vector. Research into formulation changes, modifications to the vectors themselves, and rigorous stability studies are helping to solve some of these issues.
Characterization methods for AAV vector production
It is particularly important that AAV vector manufacturing platforms include ongoing characterization of process intermediates and the final product. This allows production process to be monitored and optimized, while also providing important product quality control data. Important attributes of the AAV vector production process to consider are the virus titer, genome content ratio, and the level of aggregation formation. Various methods are available to analyze these attributes, including enzyme-linked immunosorbent assay (ELISA) for determining viral titer, transmission electron microscopy (TEM) for assessing content ratios, and dynamic light scattering (DLS) methods for measuring aggregate content.
But many of these methods can be tedious and time-consuming. For example, wash-based assays like ELISA are labor intensive and not fully amenable to scaling. No-wash assays that allow higher throughput with scalability have the potential to address this issue and drastically improve lab efficiencies.
We have developed and manufactured a line of no-wash immunoassays that detect and quantify AAV capsid (in Viral Particles per milliliter - VP/mL) utilizing AlphaLISA technology. The AlphaLISA and HTRF AAV Capsid Detection Kits are available for AAV1, AAV2, AAV3B, AAV5, AAV6, AAV8 and AAV9 serotypes, and can measure AAV particles present in cell culture media, lysis buffer and cell lysate.
For research use only. Not for use in diagnostic procedures.