Generation of “off-the-shelf” allogeneic CAR-T cells is a rapidly progressing field that is revolutionizing human health through its immense applicability and promise in cell therapy. In order to safely engineer and manufacture efficient CAR-T cells to be accepted and persist upon introduction to a patient, complex cell engineering is required.
Gene editing technologies hold promise for the progression of these next generation cell therapies; however, there is no singular approach for precise transgene knock-in and multiplex gene knockout without the generation of multiple DNA double-strand breaks or use of different CRISPR-Cas based effectors.
Beyond scissors for greater precision
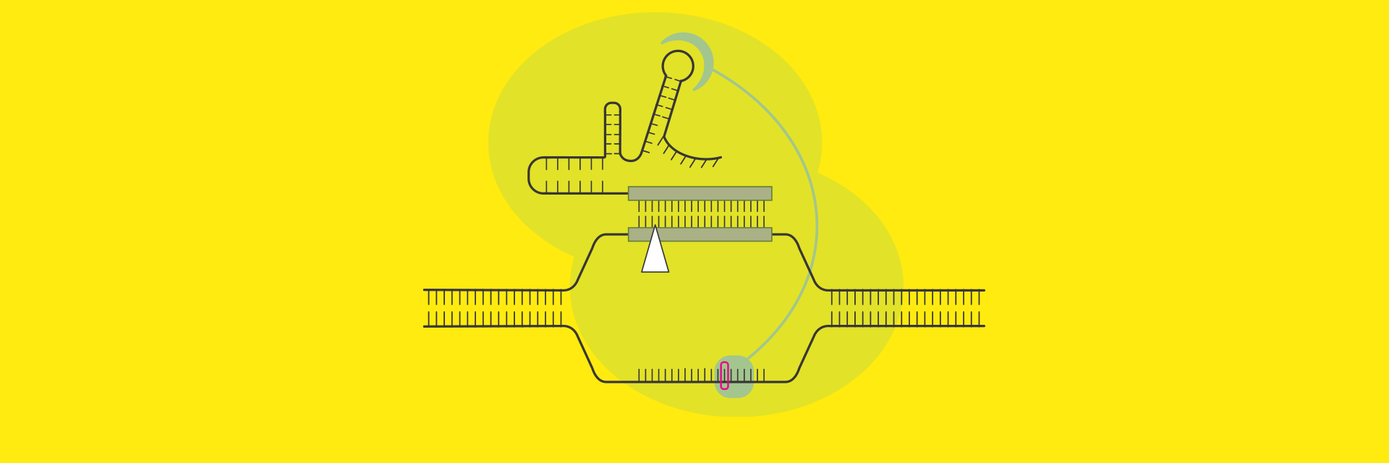
That’s why we’ve developed a technology for allogeneic CAR-T cell generation using the Pin-point™ base editing platform for simultaneous knock-in and multiple gene knockout in a single intervention.
Researchers at Revvity, in collaboration with Rutgers University and AstraZeneca Discovery Sciences, demonstrated in a recently published paper how this aptamer-mediated platform can be leveraged to modularly control base editing to induce multiplex gene knockout at three different loci, as well as DNA nicking activity to introduce targeted transgene insertion of a CAR cassette via homology directed repair at a fourth genomic location.
Compared to traditional CRISPR technologies, which create double-stranded breaks in the DNA, this novel editing system uses a modified Cas enzyme that only nicks one strand of the DNA. This allows for a more controlled approach to gene disruption and base correction.
With this proof of principle study, we demonstrate highly efficient generation of multi-edited cells, while exhibiting a significantly more favorable safety profile compared to a standard CRISPR-Cas9 approach. Beyond engineering of CAR-T cells, this method is well suited for precise and efficient creation of next generation adoptive T cell and iPSC-based therapies, as well as engineering a wide range of immune-based cell therapies for ex vivo application.
Read the full publication or find out more about the Pin-point base editing platform.
Reference:
- Porreca, I., Blassberg, R., Harbottle, J., Joubert, B., Mielczarek, O., Stombaugh, J., Hemphill, K., Sumner, J., Pazeraitis, D., Touza, J.L., Francescatto, M., Firth, M., Selmi, T., Collantes, J.C., Strezoska, Z., Taylor, B., Jin, S., Wiggins, C.M., Smith, A., Lambourne, J.J. An aptamer-mediated base editing platform for simultaneous knock-in and multiple gene knockout for allogeneic CAR-T cells generation. Molecular Therapy. 2024. https://doi.org/10.1016/j.ymthe.2024.06.033
For research use only. Not for use in diagnostic procedures.