Revvity has worked with leading virology labs including government agencies, academic labs, and pharmaceutical companies such as the Centers for Disease Control and Prevention (CDC), Food and Drug Administration (FDA), and National Institute of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID).
These labs have developed modern virology assays utilizing microplate imaging systems to increase sensitivity, efficiency, and accuracy which has led to a reduction of materials used and reduction in time to answer critical questions.
Image cytometry assays to accelerate virology research
- Microneutralization assays by detecting single-infected cells in 384- and 96-well plates
- Automated viral infectivity/titer assays based on plaque, fluorescence foci and single infected cells in 24-, 96- and 384-well plates
- Morphological quantification of plaque/foci in 24- and 96-wells within 5 minutes per plate
- EC50 & CC50 for antiviral potency and cytotoxicity in the same 384-well plate
- Cell-based antibody binding inhibition assays in 96-well plates in ~15 minutes for 3 fluorescent colors
- Automated label-free CPE assays using brightfield imaging in 96-well plates in less than 5 minutes
Faster answers with a high-throughput cell counter
- PBMC, whole blood nucleated cell count, and viability for 24 samples in less than 3 minutes
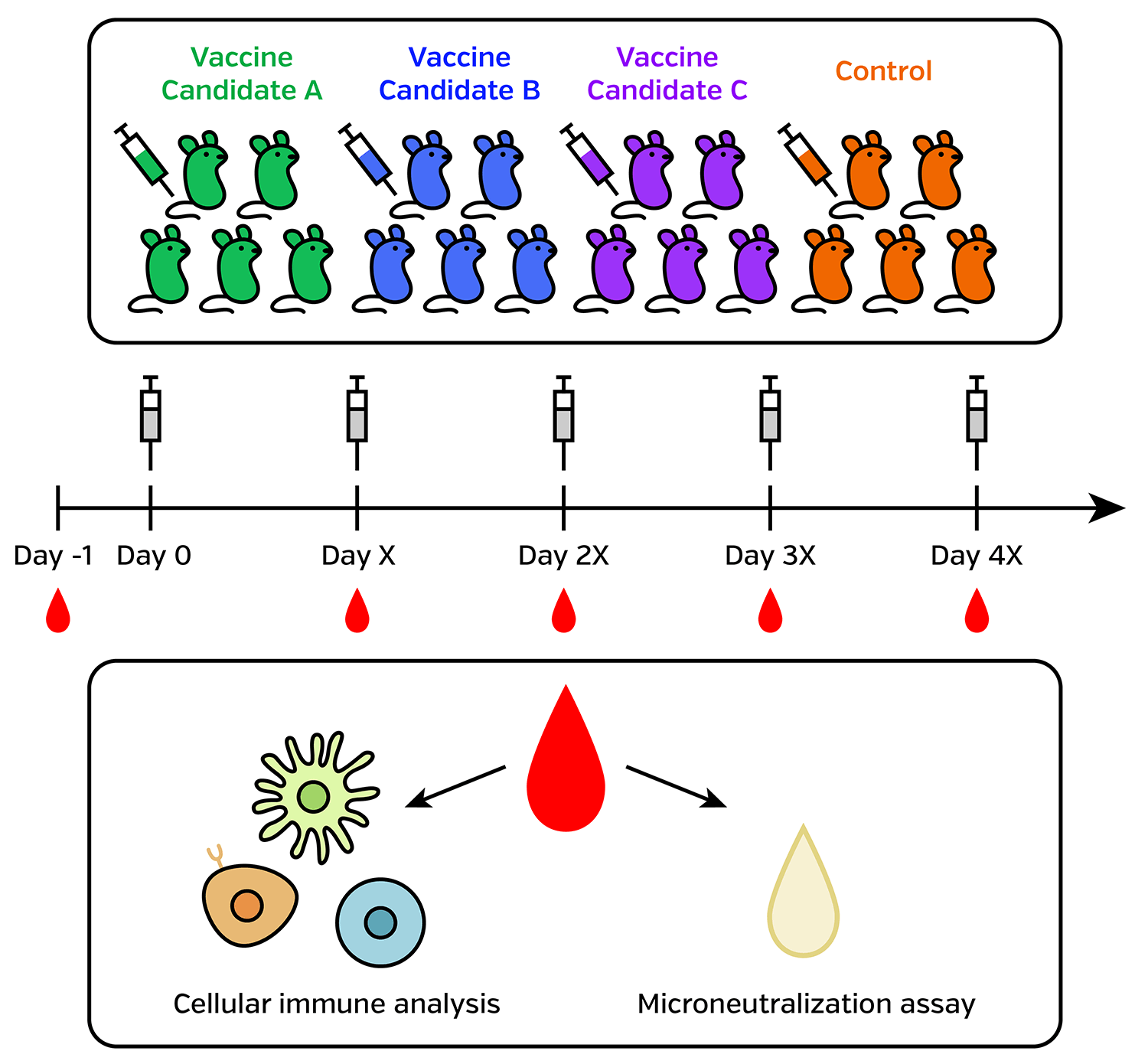
Speed up preclinical mouse model experiments
Immunogenicity testing in mouse and other small animal models are essential for vaccine development. Neutralizing antibody titer measurements are required for many animals at multiple time points. For each small animal, the amount of serum is limited. (Typical mouse blood drawn is less than 200 µl.)

Micro-neutralization assay
It is advantageous to develop micro-neutralization assays in 96-well, or even 384 well format, using an image cytometer such as the Celigo Image Cytometer.
Cellular immune analysis
To perform cellular immune analysis, obtaining viable cell count is required. Lower viability samples are typically excluded from high dimension polychromatic flow cytometry measurement.
Using a high-speed, high-throughput cell counter will increase sample processing speed, reduce delay time, and sample degradation.
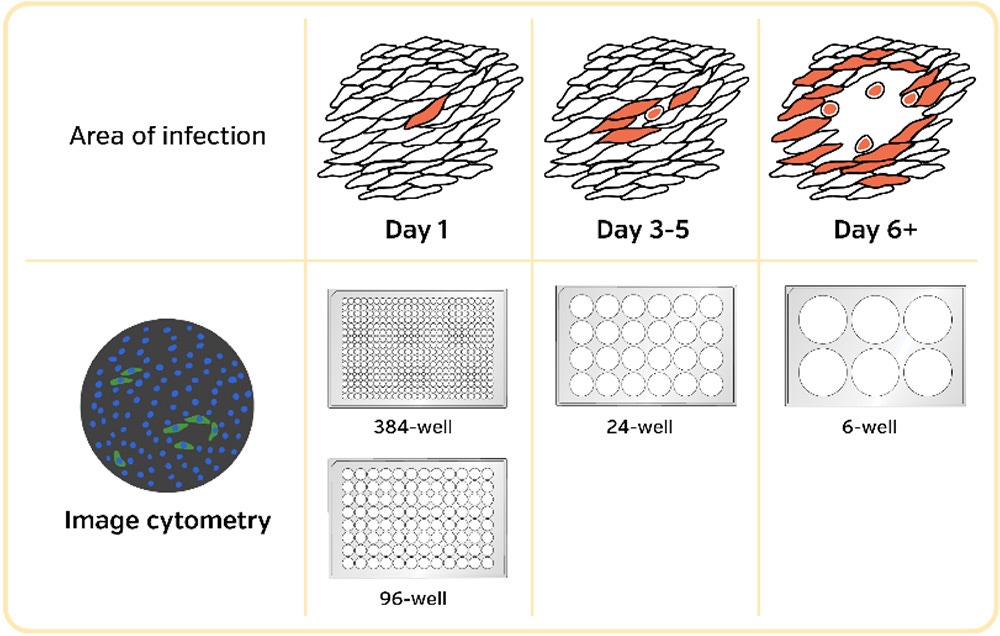
Speed up infectivity / viral titer / microneutralization experiments
Infectivity is a key measurement in the development of pathogen countermeasures. This includes antiviral small molecule screens, monoclonal antibody screens, preclinical animal models, pathogenesis, pathway discovery, and vaccine candidate evaluation.
Virus quantification involves the determination of the number of viral particles in a specific volume to obtain viral concentration.
Infectious viral concentrations are determined using two different approaches.
- Apply a serially diluted viral sample to a healthy monolayer of permissive cells and enumerate the number of discretely infected areas originated by each round of infection (Plaque forming assay)
- Apply a serial diluted viral sample to a healthy monolayer of permissive cells and document the viral dosage at which 50% cells are killed by the virus (TCID50)
For the first approach in infectious viral titer determination, the traditional manual-based visual plaque counting assays have been improved to reduce assay turn-around time, reduce variability introduced by operators, and increase sample throughput.
The Celigo image cytometer has been adapted to automate many versions of the counting-based infectivity assays in 384-, 96-, 48-, 24-, 12- and 6-well plates.
- High-content, single infected cell-based infectivity assay
- Immunofluorescence-based foci formation assay
- Immuno HRP-based foci formation assay
- Fluorescent pseudo virus infectivity assays
- Traditional lytic plaque assays
Publications using nexcelom image cytometers in virology research, vaccine development and analytical assay development for gene therapy
Many leading virology labs have utilized the Celigo image cytometer and other Revvity systems in their work, for example:
| Research Areas | Viruses |
| Pathogenesis – Dynamics of virus and human cell interaction during infection | |
| Identify cellular receptor-dependent entry pathway of virus infection “CD46 facilitates entry and dissemination of human cytomegalovirus” Stein, K.R., Gardner, T.J., Hernandez, R.E. et al. CD46 facilitates entry and dissemination of human cytomegalovirus. Nat Commun 10, 2699 (2019). doi.org/10.1038/s41467-019-10587-1 |
Human cytomegalovirus (CMV) |
| Identify genes with antiviral activity against positive-strand RNA viruses “A Short Hairpin RNA Screen of Interferon-Stimulated Genes Identifies a Novel Negative Regulator of the Cellular Antiviral Response” Jianqing Li, Steve C. Ding, Hyelim Cho, Brian C. Chung, Michael Gale Jr., Sumit K. Chanda, Michael S. Diamond mBio Jun 2013, 4 (3) e00385-13; DOI: 10.1128/mBio.00385-13 |
West Nile virus (WNV) |
| Key factor (IL-6) in viral clearance, immune cell response, lung repair “IL-6 ameliorates acute lung injury in influenza virus infection” Yang, M., Wang, C., Yang, S. et al. IL-6 ameliorates acute lung injury in influenza virus infection. Sci Rep 7, 43829 (2017). doi.org/10.1038/srep43829 “Innate Immune Response to Influenza Virus at Single-Cell Resolution in Human Epithelial Cells Revealed Paracrine Induction of Interferon Lambda 1” Irene Ramos, Gregory Smith, Frederique Ruf-Zamojski, Carles Martínez-Romero, Miguel Fribourg, Edwin A. Carbajal, Boris M. Hartmann, Venugopalan D. Nair, Nada Marjanovic, Paula L. Monteagudo, Veronica A. DeJesus, Tinaye Mutetwa, Michel Zamojski, Gene S. Tan, Ciriyam Jayaprakash, Elena Zaslavsky, Randy A. Albrecht, Stuart C. Sealfon, Adolfo García-Sastre, Ana Fernandez-Sesma Journal of Virology Sep 2019, 93 (20) e00559-19; DOI: 10.1128/JVI.00559-19 |
Influenza A virus (IAV) |
| Different host tissues respond during infection “Characterizing the Different Effects of Zika Virus Infection in Placenta and Microglia Cells” Martinez Viedma, M.P.; Pickett, B.E. Characterizing the Different Effects of Zika Virus Infection in Placenta and Microglia Cells. Viruses 2018, 10, 649. |
Zika virus |
| Study the mechanisms governing the “antiviral state” (AVS) at the mucosal sites for viral defense “IRF1 Maintains Optimal Constitutive Expression of Antiviral Gens and Regulates the Early Antiviral Response” Panda D, Gjinaj E, Bachu M, Squire E, Novatt H, Ozato K and Rabin RL (2019) IRF1 Maintains Optimal Constitutive Expression of Antiviral Genes and Regulates the Early Antiviral Response. Front. Immunol. 10:1019. doi: 10.3389/fimmu.2019.01019 |
Vesicular stomatitis virus (VSV), influenza virus |
| Vaccine development and evaluation | |
| Develop a new vaccine platform “An Influenza Virus Hemagglutinin-Based Vaccine Platform Enables the Generation of Epitope Specific Human Cytomegalovirus Antibodies” Behzadi, M.A.; Stein, K.R.; Bermúdez-González, M.C.; Simon, V.; Nachbagauer, R.; Tortorella, D. An Influenza Virus Hemagglutinin-Based Vaccine Platform Enables the Generation of Epitope Specific Human Cytomegalovirus Antibodies. Vaccines 2019, 7, 51. |
Human cytomegalovirus (CMV) |
| Microneutralization assay for human in vitro platform known as MIMIC to evaluate immune response to the vaccine candidate “A respiratory syncytial virus (RSV) F protein nanoparticle vaccine focuses antibody responses to a conserved neutralization domain” Kurt A. Swanson, Jennifer N. Rainho-Tomko, Zachary P. Williams, Lilibeth Lanza, Michael Peredelchuk, Michael Kishko, Vincent Pavot, Judith Alamares-Sapuay, Haritha Adhikarla, Sankalp Gupta, Sudha Chivukula, Scott Gallichan, Linong Zhang, Nicholas Jackson, Heesik Yoon, Darin Edwards, Chih-Jen Wei, Gary J. Nabel Science Immunology01 May 2020 |
Respiratory syncytial virus (RSV) |
| Preclinical immunogenicity and efficacy evaluation in mice for vaccine development “Purified Inactivated Zika Vaccine Candidates Afford Protection against Lethal Challenge in Mice” Baldwin, W.R., Livengood, J.A., Giebler, H.A. et al. Purified Inactivated Zika Vaccine Candidates Afford Protection against Lethal Challenge in Mice. Sci Rep 8, 16509 (2018). doi.org/10.1038/s41598-018-34735-7 |
Zika virus |
| Protein-based vaccine candidate evaluation “Antibodies Elicited by an NS1-Based Vaccine Protect Mice against Zika Virus” Mark J. Bailey, Felix Broecker, James Duehr, Fortuna Arumemi, Florian Krammer, Peter Palese, Gene S. Tan mBio Apr 2019, 10 (2) e02861-18; DOI: 10.1128/mBio.02861-18 |
Zika virus |
| Antiviral drug screening | |
| Identify druggable human proteins or factors as targets to screen existing pharmacological agents with antiviral activity “A SARS-CoV-2 protein interaction map reveals targets for drug repurposing” Gordon, D.E., Jang, G.M., Bouhaddou, M. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature (2020). doi.org/10.1038/s41586-020-2286-9 “A Large-scale Drug Repositioning Survey for SARS-CoV-2 Antivirals” bioRxiv 2020.04.16.044016; doi: doi.org/10.1101/2020.04.16.044016 |
SARS-CoV-2 virus |
| Gene therapy, vaccine development new assays | |
| Automate and speed up plaque assay for rAAV process development “Integration of Fluorescence Detection and Image-Based Automated Counting Increases Speed, Sensitivity, and Robustness of Plaque Assays” Allyson L. Masci, Emily B. Menesale, Wei-Chiang Chen, Carl Co, Xiaohui Lu, Svetlana Bergelson doi.org/10.1016/j.omtm.2019.07.007 Molecular Therapy: Methods & Clinical Development Vol. 14 September 2019 |
HSV-1 |
| “A high-throughput inhibition assay to study MERS-CoV antibody interactions using image cytometry” | MERS-CoV |
| Develop a panel of engineered reporter influenza viruses for in-depth profiling of neutralizing antibodies using 384 well microneutralization assay “A comprehensive influenza reporter virus panel for high-throughput deep profiling of neutralizing antibodies” Adrian Creanga, Rebecca A. Gillespie, Brian E. Fisher, Sarah F. Andrews, Liam Hatch, Tyler Stephens, Yaroslav Tsybovsky, Michelle C. Crank, Adrian B. McDermott, John R. Mascola, Barney S. Graham, Masaru Kanekiyo doi.org/10.1101/2020.02.24.963611 bioRxiv 2020.02.24.963611 |
Panel of influenza with fluorescence reporter |
| Monitor gene silencing induced using lentiviral vectors “Effect of Periostin Silencing on the Autophagy of Osteoblasts” Han Qin and Jun Cai Cellular Reprogramming Jun 2019.122-128. doi.org/10.1089/cell.2018.0051 |
Lentiviral vector |
For research use only. Not for use in diagnostic procedures.




























