Overview
Revvity offers a range of 96-well, 384-well and 1536-well tissue culture treated imaging plates. These include the following:
- ViewPlate™ Microplates
- Glass Bottomed ViewPlate Microplates (96-well only)
- CellCarrier™ and PhenoPlate™ (formerly CellCarrier Ultra) Microplates
High content screening plates have to fulfill several (sometimes conflicting) demands. They need to:
- Provide a suitable environment for cellular growth
- Have excellent optical quality
- Have good flatness
- Be compatible with the objective lens
The plate and objective lens must be considered in combination since they are dependent on each other. For most applications the use of black pigmented plates with a clear, flat, low, polystyrene (PS) or cyclic olefin bottom for cell culture is suitable. The choice of plate is crucial for the success of high content screening experiments. It is recommended that once a plate has been selected to fulfill all of the specific experimental requirements and has been proven to work well, it should not be changed unless it becomes necessary.
Plate considerations
Glass bottom vs. plastic bottom
Standard microtiter plates are typically available with plastic or glass bottoms.
- Tissue culture (TC) treated polystyrene plates support the binding of proteins and cell attachment and are suitable for many different applications.
- PhenoPlate (formerly CellCarrier Ultra) plates are made from a cyclic olefin polymer. This material supports cell adherence and growth but has optical properties more similar to glass, combining the better properties of polystyrene and glass bottom plates.
Plate bottom thickness
The thickness of the plate bottom has implications for the choice of the objective and the planarity of the plate bottom (in combination with the material that it is made from).
We generally recommend thin bottom (< 200 μm) plates, because they can be used with high numerical aperture (high NA) objectives. High NA objectives typically have a short working distance, but provide better resolution, and collect more light from the specimen than long working distance (long WD) objectives. Thick-bottomed plates (>250 μm) require the use of long WD objectives, which typically have a lower numerical aperture.
Please note that, independently of the objective, the minimum suitable thickness of plates is 110 μm, as focusing of plates with thinner bottoms is not supported by the laser-based autofocus system.
Plate geometry
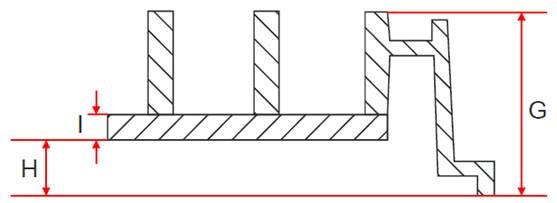
The choice of objective is not only determined by the thickness of the plate bottom (see Figure 1, value I), but also by the distance between the plate bottom and the plate rim (see Figure 1, value H). So called high-bottomed plates (H value > 300 μm) are often not suitable for an objective with a high NA or a water immersion objective as it would collide with the rim of these plates. This is the reason why for example the ViewPlate-384 microplate is not recommended for some of the high NA objectives even though the plate bottom thickness would be suitable.

Figure 1: Sketch of plate geometry, showing the values that are important to consider. These are the thickness of the plate bottom (I) and the distance between the plate bottom and the rim of the plate (H). The sum of both these values determines the suitability of plates for use with high NA objectives.
To find out if an objective can be used with a certain plate type, you can compare the value of the objectives working distance (WD) with the sum of H+I: If the WD is smaller than H+I, this combination will not work. If the WD is at least 1mm larger than H+I, you can safely assume that this combination will work. If the difference between WD and H+I is less than 1mm, you will have to check the combination at the instrument.
Planarity
The planarity of a plate bottom is influenced by its thickness and also by the material that it is made from.
- Polystyrene bottom plates can have planarity issues when very thin and/or in 96-well format. The larger well span in 96-well plate format can result in poor planarity which will affect confocal imaging; only images in the middle of the well will be sharp. In 384- or 1536-well plate format planarity is usually better even with a thin polystyrene bottom. They are therefore more suitable for HCS and HCA applications.
- Cyclic olefin plates show good planarity at various plate densities and are recommended for HCS and HCA applications.
Plate Format
96-well plates
- Advantages: Easier to use in manual mode. Better suited for long-term live cell applications as the cells are less influenced by evaporation.
- Disadvantages: Requires a higher working volume which equals higher cost. Most 96-well plates with thin polystyrene bottoms cannot be recommended due to an insufficient planarity.
384-well plates
- Advantages: The working volume is small, resulting in a cost-efficient solution for high throughput. Good planarity.
- Disadvantages: Handling several plates in a manual mode, even using 16-channel pipettes, can be highly inaccurate. Automated liquid handling devices such as the FlexDrop™ Reagent Dispenser or JANUS® Automated Workstation are recommended for dispensing and aspirating to facilitate the use of 384-well plates.
1536-well plates
- Advantages: The working volume is very small, resulting in a cost-efficient solution for high throughput. Good planarity.
- Disadvantages: Handling plates in a manual mode is not recommended. Automated liquid handling devices such as the FlexDrop™ Reagent Dispenser or JANUS® Automated Workstation are highly recommended for dispensing and aspirating to facilitate the use of 1536-well plates.
Coating
Both glass and plastic bottoms can be coated with various biomolecules to improve the confluency and adherence of cells. A variety of extracellular matrices, natural proteins and synthetic polypeptides are available for this purpose. A comparison of non-coated and collagen I coated microtiter plates is shown below. The data show that the collagen I coated surface promotes even spreading of the cells.
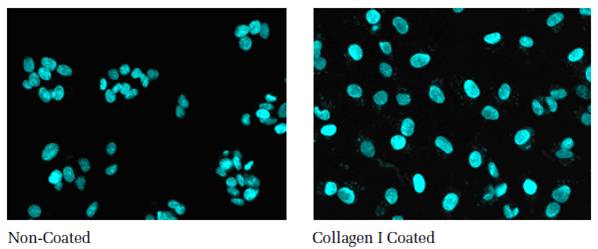
Figure 2: Comparison of cell growth on non-coated and collagen I coated Cell Carrier plates. HepG2 cells were seeded at equal densities, incubated over night at 37 °C, 5 % CO2. Following nuclear staining, the plates were imaged. Images were acquired with a 20x objective. Left: cells on the non-coated surface grow in agglomerates and in multilayers. Right: cells are evenly spread when grown on the collagen I coated surface.
Commonly used coatings include:
- Poly-lysine: a synthetic positively charged polymer, existing as two enantiomers, poly-D-lysine (PDL) and poly-L-lysine (PLL). Both are commonly used, however PDL is not degraded by cellular proteases and is therefore often the preferred choice. As poly-lysine is a synthetic protein, it does not influence the signaling pathways of the cells and is completely free of any animal contaminants. Almost all cell types will adhere to poly-lysine coated plate bottoms.
- Collagen: the most abundant protein in mammals that is found throughout the body and is a major component of the extracellular matrix (ECM). The most frequently used types of collagen for coating are collagen I and IV. Collagen type I is suitable for endothelial and epithelial cells, muscle cells and hepatocytes. Collagen type IV is the major constituent of basement membranes and offers more physiologically relevant conditions to cells as well as improving the adherence of specific cell types e.g. PC-12 (rat adrenal pheochromocytoma cell line).
3D Spheroid ULA plates
Spheroid ULA (ultra-low attachment) plates enable the use of three-dimensional (3D) cell cultures in microplate-based assays. It has been demonstrated that in many cases, three-dimensional (3D) cell culture can better recreate a biologically-relevant cellular environment, when compared to two-dimensional (2D) monolayer cell culture. The ultra-low-attachment (ULA) coating of these plates allows spheroids to form, which better simulate natural cellular interactions and better mimic in vivo microarchitecture. Our CellCarrier Spheroid ULA microplates use an ultra-low attachment surface to aid in spheroid formation. CellCarrier plates were developed for cellular imaging applications, including high content analysis and high content screening.
Plate selection table
For most applications the use of black pigmented plates with a clear, flat, low bottom for cell culture is suitable.
| PhenoPlate (formerly Cell Carrier Ultra) | ViewPlate | ||||
|---|---|---|---|---|---|
| PhenoPlate-96 | PhenoPlate-384 | ViewPlate-96 | ViewPlate-384 | ViewPlate-1536 | |
| Plate Material | Cyclic Olefin | Cyclic Olefin | Polystyrene | Polystyrene | Polystyrene |
| Well Area (mm2) | 32.1 | 10.6 | 29.2 | 10.9 | 1.8 |
| Working Volume (μL) | 50 - 350 | 25 - 100 | 80 - 350 | 10 - 100 | 4 - 12 |
| Max Volume (μL) | 425 | 145 | 360 | 135 | 12 |
| Bottom Thickness | 188 | 188 | 760 | 190 | 75 |
| Plate Bottom Height (mm) | 0.2 | 0.2 | 2.6 | 2.9 | 1.9 |
| Refractive Index of Bottom | 1.53 | 1.53 | 1.58 | 1.58 | 1.58 |
| Coatings Available | TC-treated, PDL, Collagen, ULA | TC-treated, PDL, Collagen, ULA | TC-treated, PDL, Collagen | TC-treated, PCL, Collagen | TC-treated, PDL, Collagen |
TC: tissue culture
PDL: poly-D-lysine
ULA : ultra-low attachment
For detailed information regarding the use of microplates on the Opera Phenix™ Plus High Content Screening System or Operetta™ CLS™ High Content Analysis System please contact your local Revvity Specialist.
Custom plate services at Revvity
Revvity offers custom microplate services, including bulk ordering, fast and flexible plate barcoding, biological plate coating (including poly-D-lysine, collagen, streptavidin coating, antibody coating, and other coatings on request), custom tissue culture-treatment, custom high protein binding treatment, custom sterilization of microplates, special packaging, and other microplate treatments.
For research use only. Not for use in diagnostic procedures.
The information provided above is solely for informational and research purposes only. The information does not constitute medical advice and must not be used or interpreted as such. Consult a qualified veterinarian or researcher for specific guidance or use information. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment.


































