Overview
Integrins are a family of transmembrane glycoproteins receptors that mediate the attachment between a cell and the tissues surrounding it. They are heterodimers containing two distinct chains known as the α and β subunits. A variety of different α and β subunits have been characterized in mammals and the integrins are named according to the identity of each of the subunits. For example, the integrin αvβ3 appears to play a critical role in tumor growth, metastasis and angiogenesis. IVISense™ Integrin Receptor (formerly IntegriSense) fluorescent probes specifically target the integrin αvβ3. They are comprised of a highly selective small molecule antagonist that binds integrin αvβ3 and a near-infrared (NIR) fluorophore. These probes have been developed to enable in vivo and in vitro visualization and quantification of integrin expression in tumor cells (as well as in neovasculature). The probes may help assess tumor growth, tumor angiogenesis, and treatment efficacy in animal models.

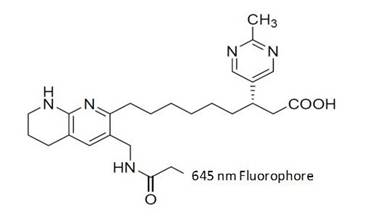
Figure 1: The integrin-targeted probe (IVISense Integrin Receptor 645) was synthesized by labeling a derivative of the αvβ3 antagonist with an amine-reactive fluorophore. It was designed in such a way to allow maximal tissue penetration and minimal absorption by physiological absorbers such as hemoglobin or water.
Products and catalog numbers
| Product | Catalog Number | Ex/Em wavelength (nm) | Molecular weight (g/mol) | Validated Experiments | Applications |
|---|---|---|---|---|---|
| IVISense Integrin Receptor 645 | NEV10640 | 649/666 | 1250 | In vivo/Ex vivo | Angiogenesis |
| Flow cytometry & In vitro microscopy | Atherosclerosis | ||||
| Cardiovascular Disease | |||||
| Inflammation | |||||
| Vascular Disease | |||||
| IVISense Integrin Receptor 680 | NEV10645 | 675/693 | 1432 | In vivo/Ex vivo | Angiogenesis |
| Flow cytometry & In vitro microscopy | Atherosclerosis | ||||
| Cardiovascular Disease | |||||
| Inflammation | |||||
| Vascular Disease | |||||
| IVISense Integrin Receptor 750 | NEV10873 | 755/775 | 1278 | In vivo/Ex vivo | Angiogenesis |
| Flow cytometry & In vitro microscopy | Atherosclerosis | ||||
| Cardiovascular Disease | |||||
| Inflammation | |||||
| Vascular Disease |
Using IVISense Integrin Receptor probe for in vivo studies
In Vivo Imaging
IVISense Integrin Receptor 645
The recommended procedure for in vivo imaging with IVISense Integrin Receptor 645 probe is administration via intravenous injection and imaging 6-24 hours post injection. Imaging may be performed as early as 3 hours with some reduction in target signal/noise. IVISense Integrin Receptor 645 will clear from tissues after approximately 6-7 days. Repeat injection and imaging may be performed every 3 days for longitudinal studies. It is recommended that a pre-injection baseline image be taken prior to reinjection and imaging, unless injections are performed every 7 days.
IVISense Integrin Receptor 680
The recommended procedure for in vivo imaging with IVISense Integrin Receptor 680 probe is administration via intravenous injection and imaging 3 – 48 hours post injection. Imaging at earlier time points (~3 hrs) is recommended when imaging the vasculature. Imaging at later time points (24-48 hrs) is recommended when imaging tumors to reduce background.
IVISense Integrin Receptor 750
The recommended procedure for in vivo imaging with IVISense Integrin Receptor 750 probe is administration via intravenous injection and imaging 24 hours post injection. Imaging may be performed as early as 6 hours with some reduction in target signal/noise. IVISense Integrin Receptor 750 will clear from tissues after ~5 days. Repeat injection and imaging may be performed every five days for longitudinal studies. It is recommended that a pre-injection baseline image be taken prior to reinjection and imaging.
| Product | Route of Injection | Mouse Dose (25 g) | Rat Dose (250 g) | Blood t 1/2 | Tissue t 1/2 | Optimal imaging time | Optimal Re-injection Time (complete clearance) | Route of Metabolism/ background tissue | FMT and IVIS settings |
|---|---|---|---|---|---|---|---|---|---|
| IVISense Integrin Receptor 645 | IV (IP) | 2 nmol | 6-20 nmol | 10 min | 48 h | 6-24 h | 6-7 d | Bladder, kidneys | FMT 635/655 |
| IVIS 640/660 with spectral unmixing | |||||||||
| IVISense Integrin Receptor 680 | IV (IP) | 2 nmol | 6-20 nmol | 10 min | 24 h | 24 h | 14 d | Kidneys, intestines | FMT 680/700 |
| IVIS 675/720 | |||||||||
| IVISense Integrin Receptor 750 | IV (IP) | 2 nmol | 6-20 nmol | 30 min | 24 h | 24 h | 4-6 d | Kidneys | FMT 750/770 |
| IVIS 745/800 |
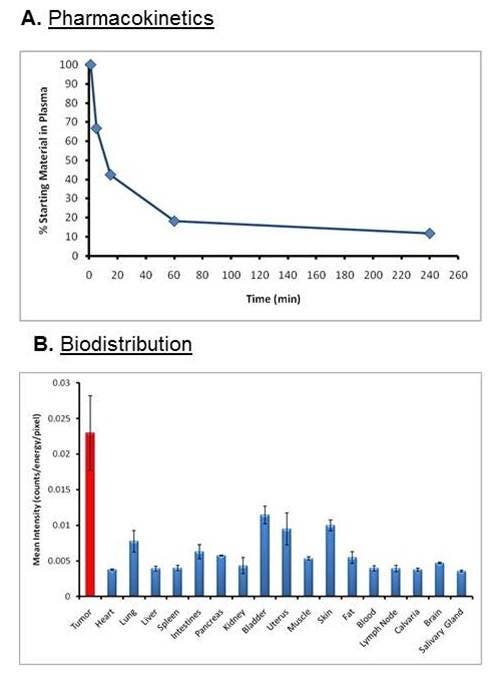
Figure 2: A) Blood pharmacokinetic profile for IVISense Integrin Receptor 645 was assessed by injecting CD-1 mice (n = 3 per time point) intravenously and collecting blood at multiple times post injection. B) Tissue samples were collected at the peak imaging time point (24 hours) and assessed by planar imaging. Mean counts/energy for each tissue were determined as a measure of tissue brightness. Tissue samples from tumors show that highest intensity of signal.
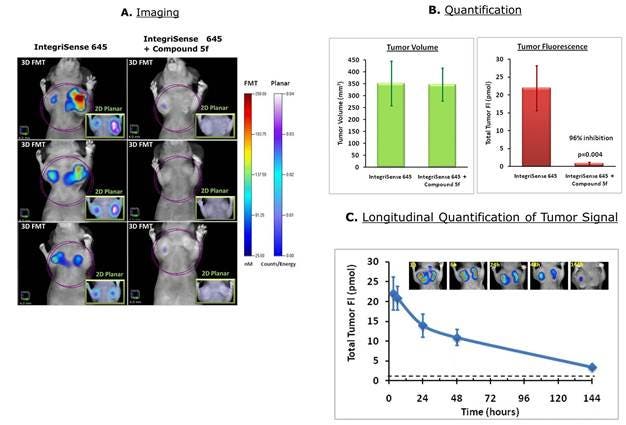
Figure 3: 4T-1 tumor-bearing mice were injected intravenously with 2 nmol IVISense Integrin Receptor 645 (formerly IntegriSense 645). A) A group of mice were pre-injected (5 min before) with the specific competitor compound 5f and imaged using fluorescence molecular tomography 24 hours by 2D planar (inserts) and 3D tomographic imaging. The panels show three representative mice per group. B) The tomographic imaging datasets were used to quantify tumor region fluorescence associated with integrin expression. The tumor volumes were determined using caliper measurements. C) The mice were imaged between 3 and 144 hours after injection with IVISense Integrin Receptor 645. The insert pictures show a representative mouse imaged at the different time points. The dashed line indicates the background fluorescence measured in control tissues.
Frozen Tissue Protocol
We have validated IVISense Integrin Receptor probes for use with frozen tissue samples. Here is a brief protocol with a recommended concentration of probe to use:
- Freeze tumor/tissue (without probe) and section 5-10 µm by cryostat.
- Incubate with 1 µM IVISense Integrin Receptor probe at 37ºC for 10-30 min.
- Wash 2x with PBS.
- Mount with anti-fade reagent.
- Fluorescence microscopy filter: Cy5 (IVISense Integrin Receptor 680, IVISense Integrin Receptor 645), or Cy5.5 or Cy7* (IVISense Integrin Receptor 750)
Using IVISense Integrin Receptor probe for in vitro studies
IVISense Integrin Receptor probes enable imaging of tumors and neovasculature in a range of oncology applications.
Flow cytometry and in vitro microscopy
We have validated IVISense Integrin Receptor probes for use with fluorescence microscopes and flow cytometers. Here is a brief protocol with a recommended concentration of probe to use:
- Culture cells in standard TC plate or chamber slide.
- Incubate cells with 0.02-0.5 μM IVISense Integrin Receptor probe for 30 min at 37 °C.
- Wash 1-2x with PBS. For flow cytometry, detach and resuspend cells in PBS
- Flow cytometry filter settings: 712/21 (IVISense Integrin Receptor 645, IVISense Integrin Receptor 680), 780/60 (IVISense Integrin Receptor 750)
- Fluorescence microscopy filter: Cy5 (IVISense Integrin Receptor 645), Cy5.5 (IVISense Integrin Receptor 680), Cy5.5 or Cy7* (IVISense Integrin Receptor 750) In Vitro Applications:
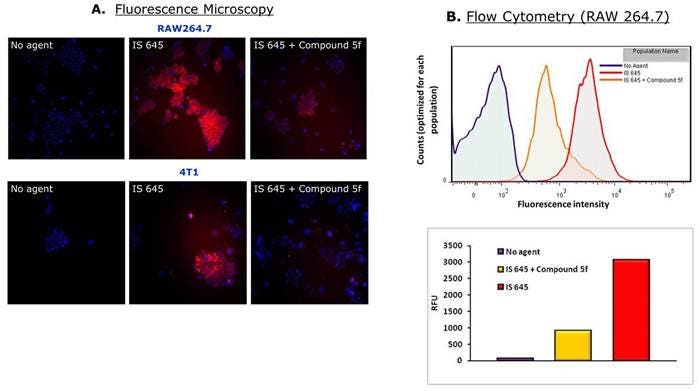
Figure 4: Raw 264.7 mouse macrophages or 4T-1 mouse breast adenocarcinoma cells were incubated with 0.25 μM of IVISense Integrin Receptor 645. Compound 5f is a specific competitor and 25 μM of it was added 15 minutes before IVISense Integrin Receptor 645 in control samples. A) Cells were then analyzed by fluorescence microscopy and B) flow cytometry using appropriate lasers and filters for detection at 645 nm wavelength. As shown in this figure, both Raw 264.7 and 4T1 cells are labeled by the IVISense Integrin Receptor 645 (red), while cells containing the specific competitor have significantly less signal.
Application notes and posters
Poster: In Vivo Quantification of Integrin-Targeted and Protease-Activated Imaging Probes in Response to Anti-Angiogenic Therapy Using Quantitative Fluorescence Tomography
FAQs
Q. Do IVISense Integrin Receptor probes cross the blood brain barrier?
A. IVISense Integrin Receptor probes have only been shown to cross into the brain in models where the blood-brain barriers are jeopardized (or compromised).
Q. Have there been any side-effects observed in the animals injected with IVISense Integrin Receptor probes?
A. None. Hundreds of animals injected with minimal to no side-effects.
Q. Can you aliquot and freeze IVISense Integrin Receptor 680/750 to make it last longer than the recommended 10 days?
A. It is not recommended
For research use only. Not for use in diagnostic procedures.




























