Overview
Hypoxia is a pathological condition where a region of the body is deprived of oxygen. Hypoxia can be associated with a variety of disease states, such as angiogenesis, pulmonary disease and inflammation. In addition, hypoxia occurs in tumors because the disorganized vascular networks cannot deliver blood borne oxygen. During hypoxia, carbonic anhydrase IX (CA IX), a cell surface enzyme, is induced and therefore, CA IX expression is a good biomarker for these disease states. IVISense™ Hypoxia CA IX 680 (formerly HypoxiSense) is a carbonic anhydrase IX (CAIX) targeted fluorescent in vivo imaging probe that can be used to image CAIX overexpression in response to hypoxia. For example, IVISense Hypoxia CA IX 680 detects the tumor cell surface expression of carbonic anhydrase 9 (CA IX) protein, which increases in cervical, colorectal, and non-small cell lung tumors. Pairing IVISense Hypoxia CA IX 680 probe with optical fluorescent imaging technology allows non-invasive imaging and quantitation of hypoxia-related changes in the cell in vivo.

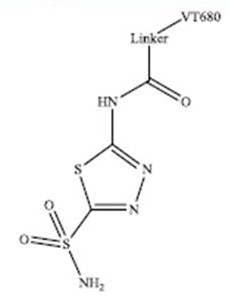
Figure 1: The CA IX targeted probe (IVISense Hypoxia CA IX 680) was synthesized by coupling the NIR (near infrared) fluorochrome IVISense 680 NHS (formerly VivoTag-680. Shown as 'VT680') to a derivative of the CA inhibitor acetazolamide.
Products and catalog numbers
| Product | Catalog Number | Ex/Em wavelength (nm) |
Molecular weight (g/mol) | Validated Experiments | Applications |
|---|---|---|---|---|---|
| IVISense Hypoxia CA IX 680 | NEV11070 | 670/685 | 1500 | In vivo/Ex vivo Flow cytometry In vitro microscopy |
Oncology Angiogenesis Inflammation Pulmonary |
Using IVISense Hypoxia CA IX 680 probe for in vivo/ex vivo studies
The general recommended procedure for in vivo imaging with IVISense Hypoxia CA IX 680 is administration via intravenous injection and imaging 24-48 hours post injection. IVISense Hypoxia CA IX 680 may also be injected intraperitoneally and imaged 24 hours post injection. IVISense Hypoxia CA IX 680 is ideally suited for detecting hypoxia-induced changes in CA IX expression.
View instructions on setting up an in vivo mouse experiment with IVISense Hypoxia CA IX 680 fluorescent probe
| Route of Injection | Mouse Dose (25 g) | Rat Dose (250 g) | Blood t 1/2 | Tissue t 1/2 | Optimal imaging time | Optimal Re-injection Time (complete clearance) | Route of Metabolism/ background tissue | FMT and IVIS settings |
|---|---|---|---|---|---|---|---|---|
| IV | 2 nmol | 6-20 nmol | 2 min | 12 h | 24 h | 6-7 d | Kidneys | FMT 680/700 IVIS 675/720 |
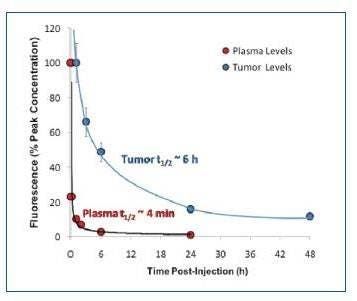
Figure 2: IVISense Hypoxia CA IX 680 clears from the bloodstream quickly, with a half-life of approximately 4 minutes. However, it accumulates within hypoxic regions in tumor tissue with a half-life of 6 hours.
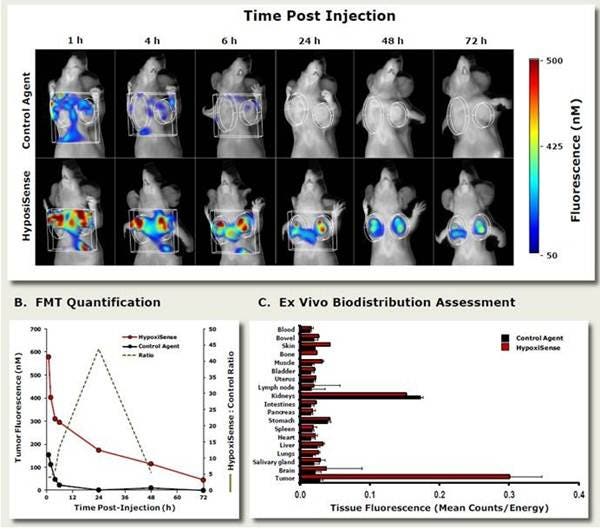
Figure 3: In Vivo Imaging. To determine the optimal imaging time for IVISense Hypoxia CA IX 680, HeLa tumor-bearing mice were injected intravenously with 4 nmol IVISense Hypoxia CA IX 680 or a control probe and imaged at various times (1, 4, 6, 24, 48, and 72h) by fluorescence molecular tomography. A) Images showing tumor signals in mice injected with either IVISense Hypoxia CA IX 680 or a control probe. B) Fluorescence tomography quantification of total pmols of fluorescence over time of IVISense Hypoxia CA IX 680 or a control probe. Dotted line shows the peak time for the best signal to noise ratio. C) Tissue samples were collected at the peak imaging time point (24 hours) and were assessed by 2D epifluorescence imaging. The mean counts/energy for each tissue was determined as a measure of tissue brightness. Tumors and kidneys showed the highest accumulation of IVISense Hypoxia CA IX 680.
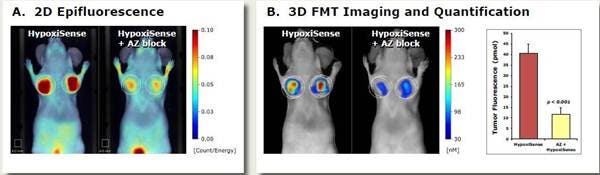
Figure 4: In Vivo Imaging. HeLa tumor-bearing mice were injected intravenously with 2 nmol of IVISense Hypoxia CA IX 680 (formerly HypoxiSense) and imaged 24 hours after injection. Control mice were intravenously injected with unlabeled acetazolamide (AZ, 10 mg/kg) CAIX inhibitor one hour prior to probe injection to test for selective binding of IVISense Hypoxia CA IX 680 to CAIX. A) 2D epifluorescence images of representative mice receiving IVISense Hypoxia CA IX 680 with or without AZ inhibitor. B) 3D fluorescence tomography and quantification of tumor fluorescence in the same mice from panel A.
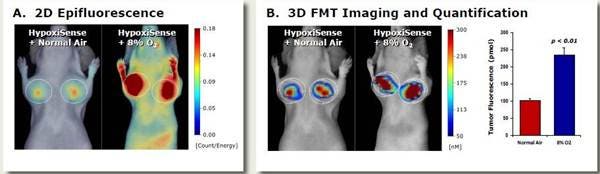
Figure 5: In Vivo Imaging. The effect of a hypoxic environment on IVISense Hypoxia CA IX 680 (formerly HypoxiSense) signal in tumors was assessed in HeLa tumor-bearing mice. Mice with HeLa tumors were exposed to either normal air (~21% O2) or placed in a sealed environmental chamber containing only 8% O2. After 24 hours, all mice were injected intravenously with 2 nmol of IVISense Hypoxia CA IX 680 and then imaged 24 hours after intravenous injection (continuing housing under either normoxic or hypoxic conditions). A) A 2D epifluorescence picture comparing the mice under the two conditions. B) A 3D tomography image and quantification of the tumor fluorescence of IVISense Hypoxia CA IX 680 under the two different conditions.
Ex Vivo Imaging:
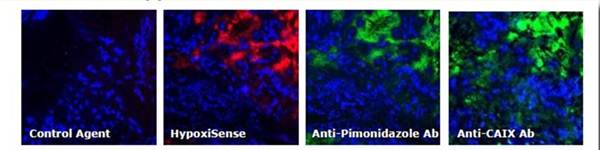
Figure 6: Ex Vivo Imaging. To determine the tumor tissue localization of IVISense Hypoxia CA IX 680 (formerly HypoxiSense) and a control probe, tumors were excised from HeLa tumor-bearing mice and fluorescence was assessed by microscopy. One hour prior to sacrifice, the mice were injected with pimonidazole (a gold standard method of labeling hypoxic tissue). Five minutes prior to sacrifice, the non-hypoxic tumor regions were visualized by injecting Hoechst perfusion agent. IVISense Hypoxia CA IX 680 /Control (red); Hoechst (blue); Anti-Pimonidazole/Anti-CAIX Abs (green).
Using IVISense Hypoxia CA IX 680 probe for in vitro studies
IVISense Hypoxia CA IX 680 enables imaging of CA-IX expressing tumor cells ex. HeLa and HT-29; HCT-116 and MDA-MB-231 (negative).
Flow cytometry and in vitro microscopy
We have validated IVISense Hypoxia CA IX 680 for use with fluorescence microscopes and flow cytometers. Here is a brief protocol with a recommended concentration of probe to use:
- Culture cells in standard TC plate or chamber slide. To induce hypoxia, transfer cells to a modular incubator chamber infused with mixed low oxygen gas for 24 h.
- Incubate cells with 1 μM IVISense Hypoxia CA IX 680 for 1 hr at 37 °C.
- Wash 2x with PBS. For flow cytometry, detach and resuspend cells in PBS.
- Flow cytometry filter settings: 712/21, Fluorescence microscopy filter: Cy5.5.
In Vitro Imaging:
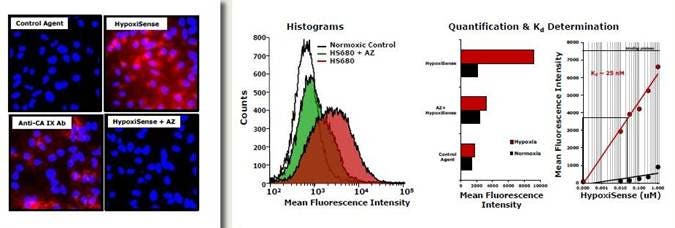
Figure 7: Tumor cells were cultured under normoxic (20% O2) and hypoxic (1.0% O2)) conditions. The cells were then incubated with 1 μM IVISense Hypoxia CA IX 680 (formerly HypoxiSense) for 1 hour at 37 °C. The red (left) indicates IVISense Hypoxia CA IX 680 signal and the blue indicates DAPI nuclear staining. For blocking with Acetazolamide (AZ): Before adding the test probes, cells were pre-incubated with 100 μM of Acetazolamide for 1 hour. Probes binding to normoxic cells were all negative by microscopy (images not shown). Binding of IVISense Hypoxia CA IX 680 and control probe to HeLa cells was then determined by fluorescence microscopy and flow cytometry (middle). An approximate Kd of IVISense Hypoxia CA IX 680 binding to tumor cells was determined by incubation with different concentrations of IVISense Hypoxia CA IX 680 and determining the concentration at which binding at half maximal (right).
FAQs
Q. Can I use IVISense Hypoxia CA IX 680 in humans?
A. No, IVISense Hypoxia CA IX 680 is intended for animal research use only, is not intended for use is diagnostic procedureand is not intended for use in humans.
Q. Is IVISense Hypoxia CA IX 680 an antibody or based on a metabolic reaction?
A. Neither, it is a modified version of the CAIX inhibitor acetazolamide.
Q. Has IVISense Hypoxia CA IX 680 been used for frozen tissue labeling?
A. This has not been tested.
Q. Can IVISense Hypoxia CA IX 680 be used with cells expressing GFP?
A. There should be no cross-reactivity between the IVISense Hypoxia CA IX 680 and GFP. The Excitation and Emission wavelengths for these two fluors are far enough apart.
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. The information does not constitute medical advice and must not be used or interpreted as such. Consult a qualified veterinarian or researcher for specific guidance or use information. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment. Users are solely responsible for complying with all relevant laws, regulations, and institutional animal care and use committee (IACUC) guidelines in their use of the information provided.




























