Overview
Bombesin receptors (BBRs) play a role in a variety of physiological systems in mammals. Three characterized receptor types (BB1R, BB2R, and BB3R) are overexpressed in several prevalent solid tumors, including prostate, breast, small cell lung, and gastrointestinal cancers. The ability to detect expression of BBRs non-invasively in animal models of cancer has provided an important tool for biomedical research. There is a bombesin family of peptides that serve as ligands for these BBRs. When the BBRs are overexpressed, the bombesin peptides serve as autocrine growth factors, signaling either calcium mobilization or proliferation in tumor. Several radio-labeled analogs of these peptides have been used for PET or SPECT imaging in preclinical cancer research as well as in imaging of patients. Cytotoxic analogs of bombesin and bombesin receptor antagonists have been explored as novel therapeutics.
IVISense™ Bombesin Receptor 680 Fluorescent Probe (formerly BombesinRSense) is a fluorescent-labeled peptide designed to bind specifically to bombesin receptors involved in cancer cell stimulation of proliferation. It is comprised of a 7-amino acid bombesin peptide analog, a near infra-red (NIR) fluorophore and a pharmacokinetic modifier to improve its plasma availability. This probe can be used to specifically target and quantify the upregulation of bombesin receptors (BBR) in vivo associated with tumor proliferation. Selectivity of probe binding was confirmed by blocking IVISense Bombesin Receptor 680-labeled human colorectal HT-29 cells with excess unlabeled native bombesin peptide. IVISense Bombesin Receptor 680 has the potential to serve as an in vivo real-time early indicator of tumor growth and chemotherapy efficacy in animal models.
Products and catalog numbers
| Product | Catalog Number | Ex/Em wavelength (nm) | Molecular weight (g/mol) | Validated Experiments | Applications |
|---|---|---|---|---|---|
| IVISense Bombesin Receptor 680 | NEV10090 | 665/691 | ~24,000 | In vivo/Ex vivo Flow cytometry In vitro microscopy | Oncology |
IVISense Bombesin Receptor 680 probe for in vivo/ex vivo studies
The generally recommended procedure for in vivo imaging with IVISense Bombesin Receptor 680 is administration via intravenous injection and imaging 24-48 hours post injection.
Repeat injection and imaging may be performed every 7 days for longitudinal studies. It is recommended that a pre-injection baseline image be taken prior to re-injection and imaging if repeat injections are to be performed less than 1 week apart.
IVISense Bombesin Receptor 680 also enables imaging of HT-29 tumors implanted subcutaneously in nude mice.
| Route of Injection | Mouse Dose (25 g) | Rat Dose (250 g) | Blood t 1/2 | Tissue t 1/2 | Optimal imaging time | Optimal Re-injection Time (complete clearance) | Route of Metabolism/ background tissue | FMT and IVIS settings |
|---|---|---|---|---|---|---|---|---|
| IV | 2 nmol | 6-20 nmol | 1.5 h | 96 h | 24 h | 6-7 d | Pancreas, kidney | FMT 680/700 IVIS 675/720 |
In Vivo Imaging Study

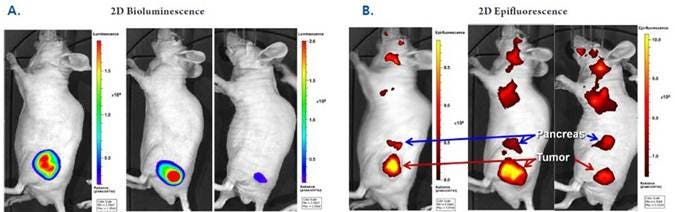
Figure 1: In Vivo Imaging: Cross-validation with Bioluminescence. NU/NU mice were implanted with human colorectal HT-29 luc tumors, injected intravenously with 2 nmol of IVISense Bombesin Receptor 680, and imaged by IVIS Spectrum CT 24 hours later. (A) Bioluminescence and (B) Epifluorescence. The same three mice per group were imaged to show tumor specific localization of IVISense Bombesin Receptor 680. Arrows indicate tumor masses (red) and pancreatic tissue (blue). Upper torso signal may be attributed to either lymph nodes or salivary glands. Tumor signal as assessed by IVISense Bombesin Receptor 680 correlates strongly with bioluminescence intensity (r2 = 0.91).
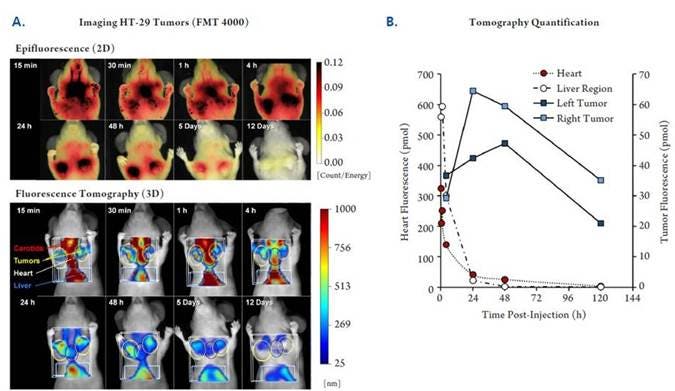
Figure 2: In Vivo Imaging: Time-course Optimization. NU/NU mice were implanted subcutaneously with human colorectal HT-29 tumors (2 tumors per mouse). When the tumors reached the desired volume, the mice were injected intravenously with 2 nmol of IVISense Bombesin Receptor 680 and imaged tomographically at times ranging from 15 minutes to 12 days. A) Shown is one representative mouse imaged at all time-points by both epifluorescence (upper panels) and fluorescence tomography (lower panels). B) Tomographic imaging datasets were used to quantify kinetic fluorescence changes in the upper liver region and the heart as well as targeted accumulation in the tumors.
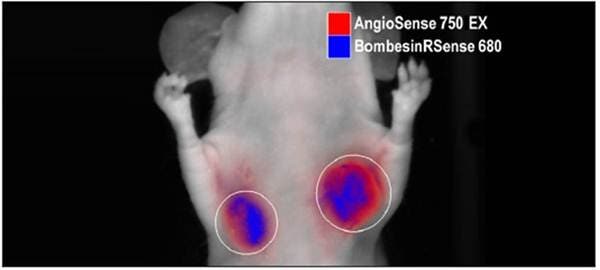
Figure 3: In Vivo Multiplex Imaging. NU/NU mice implanted subcutaneously with human colorectal HT-29 tumors were injected intravenously with 2 nmol of IVISense Bombesin Receptor 680 (formerly BombesinRSense 680) and 2 nmol of IVISense Vascular 750 (formerly AngioSense 750 EX). Epifluorescence imaging at 24 hours post intravenous injection shows overlapping intratumoral distribution of these two imaging probes.
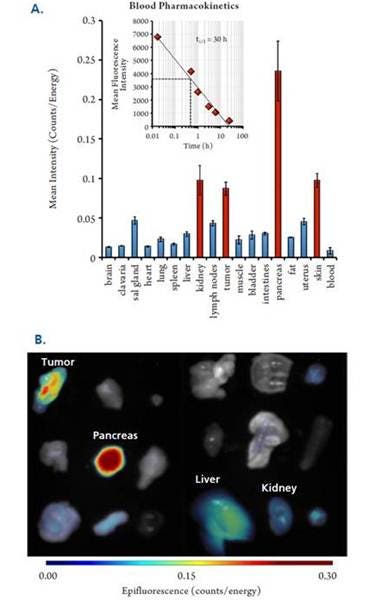
Figure 4: In Vivo Biodistribution and Pharmacokinetics. Twelve HT-29-bearing NU/NU mice were injected intravenously with 2 nmol of IVISense Bombesin Receptor 680. Blood was collected at multiple times and tissues were removed at 24 hours. A) Quantified tissue biodistribution measured by FMT epifluorescence. Inset shows plasma pharmacokinetics measured by microplate assay. B) Epifluorescence tissue images using tissues from a representative mouse.
Ex Vivo Imaging Study
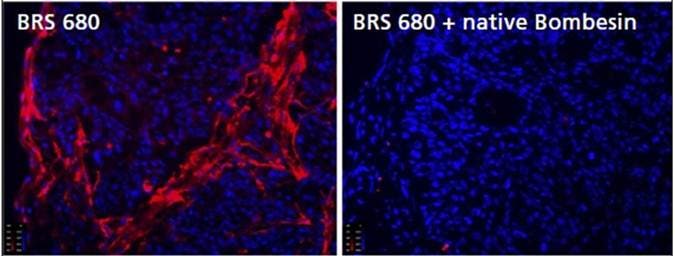
Figure 5a: Detection of BBR Expression in Frozen Tumor Tissue Sections. To assess binding specificity of IVISense Bombesin Receptor 680 (BRS 680), tumors were removed from HT-29-tumor bearing mice, and frozen sections (10 micron thickness) were incubated with 1 μM IVISense Bombesin Receptor 680 for 15 minutes at room temperature, with or without previous incubation (for 15 minutes at 37°C) with 100 μM native bombesin. The tissue was rinsed and imaged by fluorescence microscopy with IVISense Bombesin Receptor 680 signal shown in red and DAPI (shown in blue) as a nuclear counterstain.
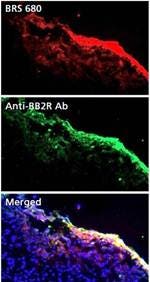
Figure 5b: Detection of BBR Expression in Frozen Tumor Tissue Sections. To confirm proper tumor localization of IVISense Bombesin Receptor 680 in vivo, HT-29 tumor bearing mice were injected with 2 nmoles of IVISense Bombesin Receptor 680. Tumors were excised 24 hours later and 10 micron sections were compared to serial sections stained with a rabbit polyclonal antibody against GRPR (BB2 receptor). Sections were imaged by fluorescence microscopy with IVISense Bombesin Receptor 680 signal shown in red and DAPI (shown in blue) as a nuclear counterstain.
Frozen Tissue Protocol
We have validated IVISense Bombesin Receptor 680 for use with frozen tissue samples. Below is a brief protocol with a recommended concentration of probe to use:
- Freeze tumor/tissue (without probe) and section 5-10 µm by cryostat. For specificity, pre-incubate sample with native bombesin 15 min prior incubation with probe.
- Incubate with 1 µM IVISense Bombesin Receptor 680 at 37ºC for 15 min.
- Wash 2x with PBS.
- Mount with anti-fade reagent.
- Fluorescence microscopy filter: Cy5.5*
IVISense Bombesin Receptor 680 for in vitro studies
Flow cytometry and in vitro microscopy
We have validated IVISense Bombesin Receptor 680 for use with fluorescence microscopes and flow cytometers. Here is a brief protocol with a recommended concentration of probe to use:
- Resuspend cells in 1x PBS. For competition study (with excess native bombesin), treat with 50 μg/ml Mitomycin C for 3 hours prior incubation with probe.
- Incubate cells with 1 µM IVISense Bombesin Receptor 680 for 5 min at 37°C
- Wash 1-2x with PBS.
- Flow cytometry filter settings: 712/21
Fluorescence micrsocopy filter: Cy 5.5
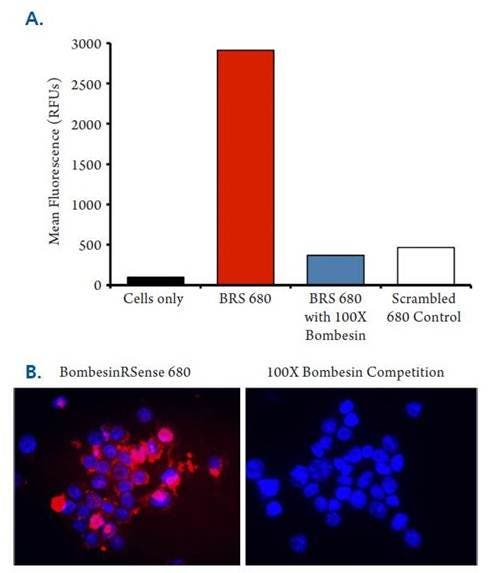
Figure 6: HT-29 cells were incubated with either 1 μM IVISense Bombesin Receptor 680 (formerly BombesinRSense 680) or a negative control probe (scrambled peptide) for 5 min at room temperature, with or without prior incubation with 100 μM of native unlabeled bombesin (15 min at 37 ˚C) to compete for binding. Cells were rinsed and imaged by both flow cytometry (A) and by fluorescence microscopy (B). Red signal is IVISense Bombesin Receptor 680 and blue signal represents DAPI nuclear counterstain.
Application notes
- Application Note: Development of a Near Infrared Fluorescent Labeled Probe for Assessing Bombesin Receptor Expression in Cancer
For research use only. Not for use in diagnostic procedures. The information provided above is solely for informational and research purposes only. The information does not constitute medical advice and must not be used or interpreted as such. Consult a qualified veterinarian or researcher for specific guidance or use information. Revvity assumes no liability or responsibility for any injuries, losses, or damages resulting from the use or misuse of the provided information, and Revvity assumes no liability for any outcomes resulting from the use or misuse of any recommendations. The information is provided on an "as is" basis without warranties of any kind. Users are responsible for determining the suitability of any recommendations for the user’s particular research. Any recommendations provided by Revvity should not be considered a substitute for a user’s own professional judgment. Users are solely responsible for complying with all relevant laws, regulations, and institutional animal care and use committee (IACUC) guidelines in their use of the information provided.




























